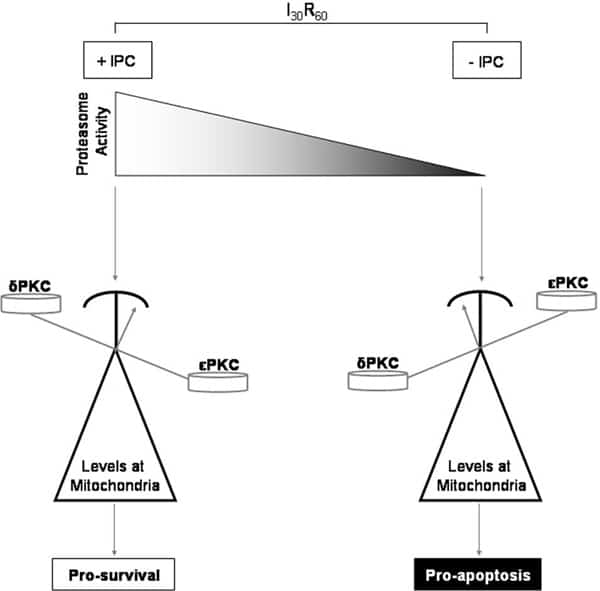
Reperfusion of blood flow to ischemic myocardium is associated with increased necrosis. Short bouts of ischemia before prolonged ischemia (ischemic preconditioning, IPC) can protect from reperfusion injury. Protein kinase-c (PKC) is a family of serine/threonine kinases that play important roles in many cellular processes including cardiomyocyte viability. Mitochondrial translocation of δPKC during cardiac reperfusion impairs mitochondrial function and initiates apoptosis. Conversely, activation of εPKC prior to ischemia maintains mitochondrial function resulting in reduced injury. Little is known about what regulates the opposing roles of these two isozymes in the pathogenesis of damage during ischemia/reperfusion (I/R). Therefore we wanted to determine the spatial and temporal relationships between δPKC and εPKC in the context of I/R and IPC to better understand their roles in cardioprotection. Using an ex vivo rat model of I/R, we determined by Western blot that IPC prior to ischemia diminished δPKC and enhanced εPKC translocation to mitochondria during reperfusion. Inhibition of εPKC with a peptide inhibitor reversed this and blocked IPC-mediated protection resulting in increased necrosis. Total levels of δPKC were decreased in response to IPC, whereas the content of εPKC did not change. Interestingly, ischemia induced a decline in proteasome activity that was protected by IPC. To better understand how cellular levels of δPKC were regulated, we blocked proteasome function using the inhibitor lactacystin. Inhibition of the proteasome prevented IPC-induced reduction of total δPKC and resulted in aberrant translocation to mitochondria with a concurrent loss in εPKC translocation. Importantly, inhibition of the proteasome prevented IPC mediated protection by restoring δPKC translocation, increasing cytochrome c release, decreasing pro-survival signaling, and increasing cellular oncosis, all of which resulted in increased tissue necrosis. Therefore, as shown in the figure, protection from I/R injury is in part dependent upon preservation of proteasome activity. Proteasome activity controls the ratio of δPKC and εPKC at mitochondria and thus anti- and pro-survival processes. Inhibition of the proteasome during ischemia tips the scale, favoring accumulation of δPKC at mitochondria resulting in the induction of apoptosis through cytochrome c release (black). In contrast, IPC prior to ischemia protects proteasome function increasing δPKC degradation allowing for εPKC accumulation at mitochondria and protection (white). Regulation of proteasome activity to control the ratios of two opposing PKCs represents a novel way to control cellular viability.
Life Sciences 2007 (2007) Proc Life Sciences, C47
Research Symposium: Proteasomal alterations in the ratios of two novel PKC isoforms regulates myocardial injury following ischemia/reperfusion
E. N. Churchill1, L. I. Szweda2, D. Mochly-Rosen1
1. Chemical and Systems Biology, Stanford University, Stanford, CA, USA. 2. Oklahoma Medical Research Foundation, Oklahoma City, OK, USA.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.