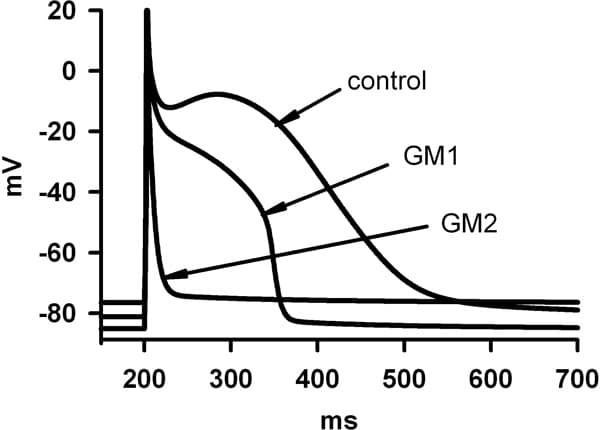
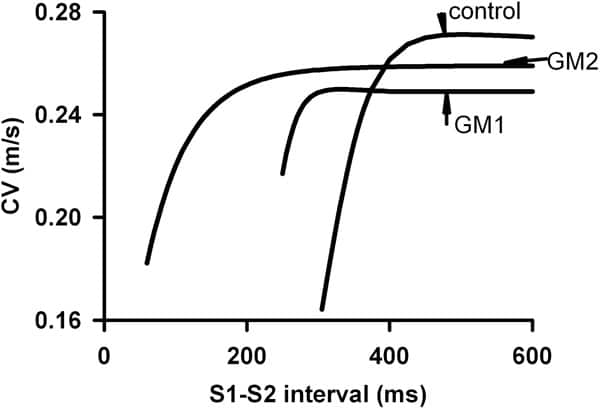
Proarrhythmogenic effects of two gene mutations were evaluated computationally on genesis and maintenance of familial atrial fibrillation (AF). These were the Kir2.1 V93I mutation (GM1) and the KvLQT1 S140G mutation (GM2), that produce gain-in-function of the inward rectifier K+ current, IK1, and of the slow delayed rectifier K+ current, IKs respectively. Electrophysiological changes induced by GM1 in IK1 (Xia et al. [1]) and GM2 in IKs (Chen et al. [2]) were incorporated into a cellular model of the human atrial action potential [3]. Changes in action potential duration (APD), APD restitution (APDR) and effective refractory period (ERP) were quantified by standard S1-S2 protocols. Rate-dependence of intra-atrial conduction velocity (CV) and the temporal vulnerability (VW) were computed in 1D models. Using 2D models, the dynamical behaviour of reentrant waves was characterised by tip meander, stability and lifespan. With both GM1 and GM2, atrial APD and ERP were remarkably abbreviated. The computed APD decreased from 323 ms in control to 258 ms (30% reduction) with GM1 and 22.2 ms (93% reduction) with GM2 (Figure 1). APDR was flattened: maximal slopes were 2.3, 0.46 and 0.011 in control, GM1 and GM2 respectively. Intra-atrial CV with a solitary wave was decreased from 0.27 m/s in control to 0.255 m/s with GM1 and 0.259 m/s with GM2. Both mutations facilitated high rate atrial excitation/conduction – the measured maximal rate of atrial excitation/conduction was 196, 240 and 1000 beats/min for control, GM1 and GM2, respectively (Figure 2). Both mutations reduced the VW (from 16.5 ms in control to 13.7 ms with GM1 and 7.59 ms with GM2), but sustained reentrant excitation. In 2D simulations, reentry self-terminated in control (LS, ~1400 ms), but persisted with GM1 and GM2. With GM1, a reentrant spiral wave degenerated into multiple stable spiral wavelets with a maximum of seven co-existing wavelets. With GM2, a reentrant spiral wave presented as a stable and persistent mother rotor with its tip meandering in a small tissue region. We conclude that both mutations reduce atrial APD and slow down intra-atrial conduction (producing a shortened wavelength of atrial excitation waves), which helps to stabilise atrial reentry and enable co-existence of multiple wavelets. Though the tissue’s vulnerability to genesis of reentry is decreased by both mutations, the lifespan of reentry is remarkably increased leading to persistent AF. This study provides insights into understanding the genesis and maintenance of chronic AF in patients with the two gene mutations.
Life Sciences 2007 (2007) Proc Life Sciences, PC146
Poster Communications: Proarrythmogenic effects of Kir2.1 and KvLQT1 familial gene mutations in human atrium: a computational study
S. Kharche1, J. Stott1, M. R. Boyett2, H. Zhang1
1. School of Physics and Astronomy, University of Manchester, Manchester, United Kingdom. 2. School of Medicine, University of Manchester, Manchester, United Kingdom.
View other abstracts by:
Figure 1. Action potential profiles in control GM1 and GM2.
Figure 2. CV restitution curves for control GM1 and GM2.
Where applicable, experiments conform with Society ethical requirements.