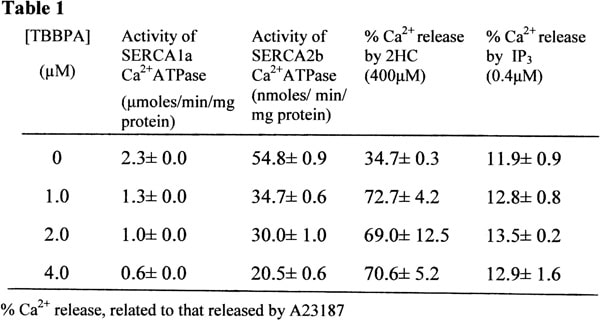
Tetrabromobisphenol A (TBBPA) is currently the most commonly employed type of the brominated flame retardant (BFR), used to treat plastics and furnishings in order to reduce their flammability. BFRs including TBBPA and their metabolites have been shown to bio-accumulate to relatively high levels within humans as result of repeated environmental exposure [1]. Recent studies have also shown that low concentrations of TBBPA can modulate a number of cell signalling processes [2]. Using Ca,2+ATPase activity measurements as described in [3], the data in table 1 shows that TBBPA is a very potent inhibitor of both SERCA1a and SERCA2b Ca2+ATPases (IC50 values of 1.7 and 2.3μM, respectively). Subsequent studies have indicated that Ca2+ pump inhibition is due to TBBPA decreasing its affinity for Ca2+. Thus TBBPA could potentially affect Ca2+ homeostasis processes leading to cellular dysfunction. In order to assess whether other commonly found intracellular Ca2+ transporters were also affected by TBBPA, its effects on both the inositol-1,4,5-trisphosphate receptor (IP3R) and Ryanodine receptor (RyR) was also investigated. The effects of TBBPA on the function of the RyR was explored by assessing whether TBBPA could affect the opening of the channel by 2-Hydroxycarbazole (2HC), a potent caffeine-like agonist [4]. Ca2+ release via RyR was observed from muscle SR vesicles using suboptimal concentrations of 2HC (400μM). In Table 1 the data shows that pre-incubation of the vesicles with TBBPA at concentrations between 1-4μM greatly enhanced 2HC-induced Ca2+ release through the RyR. This result therefore indicates that TBBPA sensitizes the RyR to release more Ca2+ upon stimulation with 2HC. It is yet to be determined whether this increased Ca2+ release is due to an increase in the duration of the open state of the channel or due to a higher conductance state. The activation of the IP3R was monitored by the addition of IP3 to cerebellar microsomes pre-accumulated with Ca2+ and the changes in calcium measured using Fluo-3[5]. As shown in the table the extent of Ca2+ release in cerebella microsomes induced by IP3 was not significantly affected, in the presence of up to 4μM TBBPA. The results presented here would indicate that low concentrations of TBBPA could have dramatic affects upon Ca2+ signalling processes within cells by abnormally elevating intracellular Ca2+ levels, which could ultimately lead to cell dysregulation and death. Our studies would indicate that TBBPA should be treated as a potential environmental ‘endocrine disrupter’.
Life Sciences 2007 (2007) Proc Life Sciences, PC270
Poster Communications: The brominated flame retardant,Tetrabromobisphenol A, inhibits SERCA Ca2+ pumps and sensitizes the Ryanodine receptor
O. A. Ogunbayo1, H. Ho Tin Wu1, F. Michelangeli1
1. Biosciences, university of Birmingham, Birmingham, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.