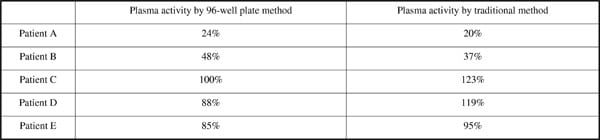
Von Willebrand Factor (vWF) mediates platelet adhesion and thrombus formation at sites of vascular injury, and also acts as a carrier of procoagulant factor VIII in circulating blood; both are essential for haemostasis. Von Willebrand disease (vWD) is a common inherited human disorder of haemostasis, where the underlying cause of bleeding is related to a quantitative or qualitative abnormality of vWF 1. Plasma vWF activity can be determined by the ability of patient plasma to support a ristocetin-induced aggregation of formalin-fixed platelets 2. Patients with a vWD have a reduced activity in ristocetin cofactor assays and these are used routinely for diagnostic purposes 3. Here we have developed a 96-well plate method for the rapid, small volume determination of vWF activity. Reference control and patient plasma samples were diluted to six concentrations, and plated in duplicate volumes of 10µl into the individual wells of a 96-well plate. Lyophilised formalin-fixed platelets were reconstituted in 5ml tris-buffered saline and incubated in volumes of 80µl with the plasma samples for 3 minutes. Ristocetin (10µl; 1mg/ml final concentration) was then added to each well to stimulate aggregation, and the plate was agitated on a heated plate shaker at 37°C. The plate was read on a plate reader at 595nm at 4, 8, 12 and 16min. The absorbance at each time point was then converted to percentage aggregation, using absorbances of vehicle-treated wells as control values. As the reference control plasma samples were supplied with known vWF activities, the activities of the patient samples could be determined by direct comparison. Table 1 shows the activity for each plasma sample from our 96-well plate assay compared to their activities determined by The Royal London Hospital Haematology Department in their standard vWF ristocetin cofactor assay. Normal physiological activity falls between 50% and 150%. We have determined that a 96-well plate based method provides a quick and accurate method for calculating vWF activity in patients. This provides an opportunity for the improvement of diagnostics by providing a higher throughput assay which maintains the necessary accuracy.
Life Sciences 2007 (2007) Proc Life Sciences, PC390
Poster Communications: Use of a 96-well plate method for the determination of ristocetin cofactor activity in patient plasma samples
N. Pearson1, T. Warner1, K. Langley2, J. Pasi3, P. MacCallum4
1. William Harvey Research Institute, Barts and the London, London, United Kingdom. 2. Haemostasis Laboratory, The Royal London Hospital, London, United Kingdom. 3. Institute of Cell and Molecular Science, Barts and the London, London, United Kingdom. 4. Wolfson Institute of Preventive Medicine, Barts and the London, London, United Kingdom.
View other abstracts by:
Table 1. Activity of the plasma samples determined by 96-well aggregometry compared to activity determined by traditional Born aggregometry
Where applicable, experiments conform with Society ethical requirements.