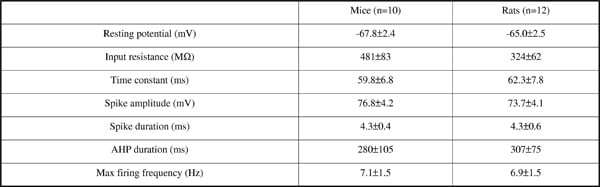
Sympathetic preganglionic neurones in both the cat in vivo (1) and rat in vitro (2) possess distinctive electrophysiology with large amplitude, long duration action potentials, a biphasic afterhyperpolarisation, pronounced transient rectification and anomalous rectification. We are interested in taking advantage of the genetic tractability of mice to examine the functional role of genes expressed in SPN, however to date no recording from mouse has been reported, thus we have sought to compare the properties of mouse and rat SPN in spinal cord slices in vitro. Mice (n=6, p8-12, C57/Bl) and rats (n=7, p8-12, Wistar) were deeply anaesthetised with halothane, decapitated and the spinal cord was removed. Transverse slices (300μm) of mid-thoracic spinal cord were cut in aCSF at 4°C. After recovering for an hour at 36°C, visualised whole-cell patch clamp recordings were made at room temperature (18-20°C) from neurones in the lateral horn. The pipette solution contained KGluconate-130; KCl-10; NaCl-10; MgCl2-2; HEPES-10; ATP-2; GTP-0.2 with Lucifer yellow (1mg/ml) to enable morphological identification. Voltage responses to injected current were used to study the active and passive membrane properties. The membrane time constant was calculated by fitting multiple exponentials to small hyperpolarising responses to current steps (<5mV). Data reported as mean±SE and statistical significance determined using unpaired t-test (at P<0.05). Recordings from mouse (n=10) and rat (n=12) SPN showed similar properties both qualitatively (presence of transient rectification, anomalous rectification, action potential shape) and quantitatively in their active and passive membrane properties (table 1). There were no significant differences between the species in any of the measured parameters. Mouse SPN exhibit the same characteristic profile of active and passive membrane properties that have been reported previously for cat (1) and rat (2). Thus the mouse should make a useful model to profile the molecular basis of these characteristic membrane properties and examine their functional role in determining the patterning of the sympathetic outflow.
Life Sciences 2007 (2007) Proc Life Sciences, PC415
Poster Communications: Comparison of the electrophysiological properties of neonatal mouse and rat sympathetic preganglionic neurones in vitro
A. O. Stalbovskiy1, J. F. Paton1, N. Balthasar1, A. E. Pickering1, 2
1. Physiology, University of Bristol, Bristol, United Kingdom. 2. Anaesthesia, University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Table 1
Where applicable, experiments conform with Society ethical requirements.