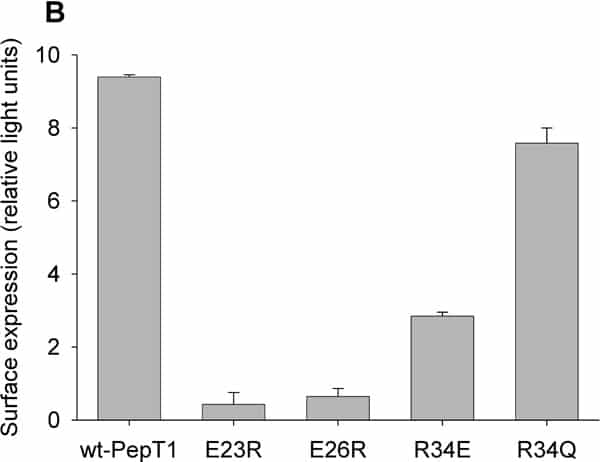
PepT1 mediates the intestinal absorption and renal re-absorption of di- and tri-peptides and a great number of therapeutically active compounds (reviewed in Daniel et al., 2006). A 12 transmembrane domain (TMD) topology was proposed originally (Fei et al., 1994) and largely confirmed by epitope mapping, with the exception of TMDs 1&2 (Covitz et al., 1998). Recently, re-analysis of the sequence and homology modelling of PepT1 have suggested that TMD1 may have been mis-assigned (Meredith & Price, 2007). Rather than being amino acid residues 7-25, TMD1 is predicted by MEMSAT-3 to be formed from residues 24-42. Homology modelling suggests TMD1 will be tilted relative to the bilayer and therefore to be membrane spanning will need to be longer, and would be formed by residues 13-42. This re-assignment would put the conserved charged residues E23, E36 and R34 into TMD1; interestingly, E23 and E26 are conserved in one of the signature motifs for the dipeptide/tripeptide permease superfamily PTR2 (Daniel et al., 2006), while R34 is strongly conserved in higher organisms. Here we test whether these residues play a functional role in rabbit PepT1. The mutants E23R, E26R, R34E and R34Q were generated using a PCR based protocol on rabbit PepT1-FLAG epitope tagged template (wt-PepT1), and expressed in Xenopus oocytes. Both membrane expression and the transport properties were determined by luminometry and uptake of 0.4μM [3H]-D-Phe-L-Gln respectively using techniques previously described (Panitsas et al., 2006). Data are means ± SEM. As can be seen in Figure 1A, the uptake of D-Phe-L-Gln into oocytes expressing E23R-, E26R-, R34E- or R34Q-PepT1 at pHout 5.5 was not significantly greater than that of non-injected oocytes, in contrast to those expressing wt-PepT1 (p>0.05, Student’s t-test, n=5 oocytes per data point, representative of up to 4 preparations). When the level of protein surface expression was quantified by luminometry (Figure 1B), it is clear that E23R- and E26R-PepT1 are not expressed at the membrane, whereas wt-PepT1, R34E- and R34Q-PepT1 were significantly expressed (p<0.001, n=12-18 oocyes per data point). These findings suggest that the residues mutated are crucial for functional rabbit PepT1 expression. E23 and E26 are implicated in proper protein folding/trafficking, as when mutated neither was expressed, whereas R34 is important for PepT1 function, as there was no dipeptide uptake despite membrane expression.
Life Sciences 2007 (2007) Proc Life Sciences, PC538
Poster Communications: Identification of functionally inportant residues in transmembrane domain 1 (TMD1) of rabbit PepT1, expressed in Xenopus oocytes
R. Spacie1, K. McCurdy1, M. Pieri1, D. Meredith1
1. Dept of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom.
View other abstracts by:
Figure 1: (A) PepT1-dependent uptake of [3H]-D-Phe-L-Gln and (B) transporter surface expression in Xenopus oocytes expressing wt-PepT1 E23R- E26R- R34E- or R34Q-PepT1.
Where applicable, experiments conform with Society ethical requirements.

![Figure 1: (A) PepT1-dependent uptake of [3H]-D-Phe-L-Gln and (B) transporter surface expression in Xenopus oocytes expressing wt-PepT1 E23R- E26R- R34E- or R34Q-PepT1.](https://static.physoc.org/app/uploads/2019/01/22203537/PC538_A.jpg)