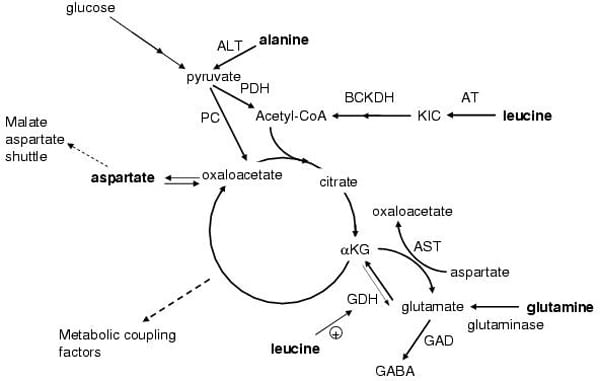
Specific amino acids are known to acutely and chronically regulate insulin secretion from pancreatic beta-cells in vivo and in vitro. Mitochondrial metabolism is crucial for the coupling of amino acid and glucose recognition to exocytosis of insulin granules. This is illustrated by in vitro and in vivo observations that mitochondrial dysfunction severely impairs insulin secretion. Mitochondria generate ATP, which is the main coupling messenger in insulin secretion, and other coupling factors, which serve as sensors for the control of the exocytotic process [1]. Numerous studies have sought to identify the factors that mediate the key amplifying pathway over the Ca2+ signal in nutrient-stimulated insulin secretion. Predominantly, these factors are nucleotides (ATP, GTP, cAMP, and NADPH), although metabolites have also been proposed, such as long-chain acyl-CoA derivatives and glutamate. This scenario further highlights the importance of the key enzymes or transporters, e.g., glutamate dehydrogenase, the aspartate and alanine aminotransferases and the malate-aspartate shuttle in the control of insulin secretion, see Figure 1 [2]. The coupling of cytosolic glycolytic NADH production with the mitochondrial electron transport chain is crucial for pancreatic beta-cell function and energy metabolism. The activity of lactate dehydrogenase in the beta-cell is low, thus glycolysis-derived electrons are transported towards the mitochondrial matrix by a NADH shuttle system, which in turn regenerates cytosolic NAD+. Mitochondrial electron transport then produces ATP, the main coupling factor for insulin secretion. Aralar1, a Ca2+-sensitive member of the malate–aspartate shuttle expressed in beta-cells, has been found to play a significant role in nutrient-stimulated insulin secretion and beta-cell function. Increased capacity of Aralar1 enhances the responsiveness of the cell to glucose. Conversely, inhibition of the malate–aspartate shuttle results in impaired glucose metabolism and insulin secretion. Recent work has described potentiating or attenuating activities of various amino acids on insulin secretion, mitochondrial membrane potential and NADH production in Aralar1-overexpressing beta-cells. This work has provided evidence for a central role of Aralar1 in the regulation of nutrient metabolism in the beta-cell. Therefore, amino acids may play a direct or indirect (via generation of putative messengers of mitochondrial origin) role in insulin secretion and action. Furthermore in periods of fasting or starvation, amino acid release from skeletal muscle (primarily L-glutamine and L-alanine) may modulate glucagon release from pancreatic alpha-cells, which subsequently may influence insulin secretion from beta-cells. Dietary amino acids may also stimulate incretin release, e.g., GLP-1, from intestinal L-cells and therefore stimulate insulin secretion via indirect mechanisms. Work in my laboratory has demonstrated that amino acid metabolism in the beta cell is essential for acute stimulation of insulin secretion in the presence of stimulatory concentrations of glucose [3, 4]. The key roles of amino acid metabolism in the beta cell will be illustrated using the examples of L-alanine and L-glutamine. In addition, after chronic exposure, amino acids may influence gene expression in the beta-cell, which subsequently alters levels of insulin secretion. Expression profiling of clonal BRIN-BD11 beta cells was performed using oligonucleotide microarray analysis. Culture for 24 hours with 10 mM L-glutamine compared to 1mM resulted in substantial changes in gene expression including many genes involved in cellular signaling, metabolism, gene regulation and the insulin secretory response. Subsequent functional experiments confirmed that L-glutamine increased the activity of the Ca2+ regulated phosphatase calcineurin. L-glutamate was released into the extracellular medium at high rates from beta cells exposed to L-glutamine [5]. These observations indicate important long-term effects of L-glutamine in regulating beta cell gene expression, signaling and secretory function.
Life Sciences 2007 (2007) Proc Life Sciences, SA38
Research Symposium: Amino acid metabolism, insulin secretion and diabetes
P. Newsholme1, 2, L. Brennan1, 2, K. Bender1, 2, A. Kiely1, 2
1. School of Biomolecular and Biomedical Science, UCD Dublin, Dublin, Ireland. 2. Conway Institute, UCD Dublin, Dublin, Ireland.
View other abstracts by:
Fig. 1 Schematic describing metabolism of selected amino acids and the related production of metabolic stimulus-secretion coupling factors involved in insulin release. The pathway of glutamine metabolism via glutaminase GDH and entry into the TCA cycle (glutaminolysis) is illustrated along with essential points of amino acid interaction with glutamine and glucose metabolism.
Where applicable, experiments conform with Society ethical requirements.