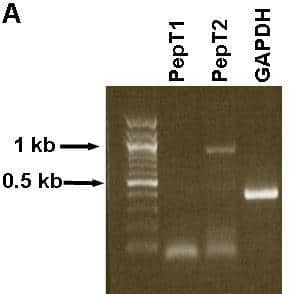
It is known that the surfactant-secreting alveolar type II (ATII) pneumocytes can mediate peptide transport and express the proton-coupled peptide transporter PepT2 (SLC15A2, reviewed in Meredith & Boyd 2000). The human A549 cell line is believed to be derived from ATII cells, based on shared morphological and biological features including the expression of surfactant genes. The aim of this study was to investigate peptide transporter expression and function in A549 cells. A549 cells were cultured in F12 medium with 5% CO2 at 37°C. RNA preparation and RT-PCR were performed using primer sequences for PepT1 and PepT2 and methods as previously reported (Liu et al. 1995; Meredith et al. 2002). Caco-2 cells were used as a positive control for the PepT1 PCR reaction. A549 cells were fixed in 4% paraformaldehyde/PBS and embedded in LRGold resin for electron microscopy and immunogold detection of PepT1 and PepT2 with polyclonal antibodies for PepT1 (Panitsas et al. 2006) and PepT2 (Autogen Bioclear UK). In addition, transport experiments were performed on A549 cells grown to a confluent monolayer on 6-well plates. The culture medium was replaced with 0.4µM [3H]-D-Phe-L-Gln in pH5.5 Krebs, in the absence or presence of 25mM Gly-L-Gln. RT-PCR studies showed that A549 cells expressed PepT2, but not PepT1 (Figure 1A) and the identity of the PepT2 band was confirmed by sequencing. In positive control Caco-2 cells PepT1 expression was confirmed while a negative control RT-PCR (no RNA template) gave no bands (data not shown). Despite the presence of PepT2 RNA, A549 cells did not show any evidence of mediated transport for the model dipeptide [3H]-D-Phe-L-Gln (Figure 1B). Under the same conditions, Caco-2 cells showed PepT1 mediated transport (data not shown). However, this apparent anomaly was explained when the distribution of PepT2 was examined by immunogold electron microscopy, as most of the immunogold labelling was found to be intracellular. In agreement with the RT-PCR results, no specific immunogold labelling for PepT1 was observed (data not shown). In conclusion, like the ATII cells, A549 cells express PepT2 but not PepT1. Most of the PepT2 protein in A549 cells was detected intracellularly and no dipeptide uptake was measured, in contrast to ATII cells in which PepT2 activity at the plasma membrane has been demonstrated (Groneberg et al. 2001). Although the lack of PepT1 expression in A549 cells argues against the idea of being able to target lung cancer cells with a PepT1-specific anticancer prodrug, we will investigate other lung cancer cell lines in future studies.
University of Cambridge (2008) Proc Physiol Soc 11, C97
Oral Communications: Expression of SLC15 family proton-coupled peptide transporters in the human A549 lung cell line.
M. Paul-Smith1, M. Pieri1,2, H. Christian1, D. Meredith2
1. Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom. 2. Department of Life Sciences, Oxford Brookes University, Oxford, United Kingdom.
View other abstracts by:
Figure 1A: RT-PCR for PepT1 PepT2 and GAPDH in A549 cells (1% TAE agarose gel).
Figure 1B: Uptake of [3H]-D-Phe-L-Gln into A549 cells in the absence or presence of 25mM Gly-L-Gln (n=3 wells per condition 1 hr uptake).
Where applicable, experiments conform with Society ethical requirements.

![Figure 1B: Uptake of [3H]-D-Phe-L-Gln into A549 cells in the absence or presence of 25mM Gly-L-Gln (n=3 wells per condition 1 hr uptake).](https://static.physoc.org/app/uploads/2019/01/22203515/C97B.jpg)