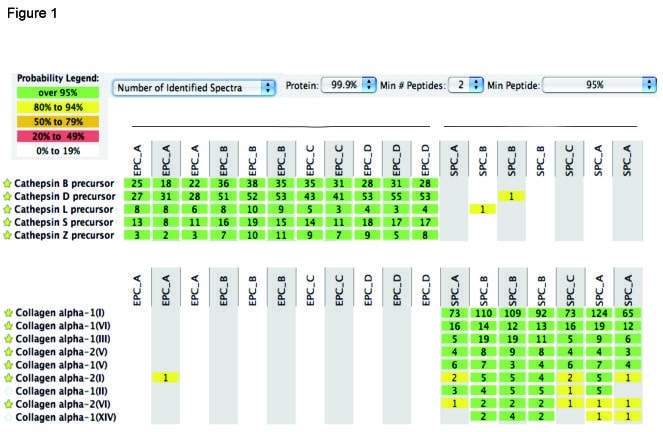
Background. Recent studies on cardiovascular progenitors have led to a new appreciation of their secretome, as paracrine factors may support the regeneration of damaged tissues. Methods and Results. Here we utilized a shot-gun proteomics strategy to define endothelial and smooth muscle progenitors (EPCs and SPCs) based on their distinct proteomic profiles in the secretome. Our data confirms previous results from microarray experiments (Urbich C et al. 2005) that EPCs express high levels of cathepsins (Fig 1). In contrast, no cathepsins were detected in SPCs conditioned medium. SPCs also showed attenuated production of proteolytic enzymes and inflammatory cytokines, but secreted extracellular matrix molecules such as a variety of collagen chains and fibronectin. Compared to their mature smooth muscle counterparts, SPCs produced different isoforms of matrix proteins as evidenced by the truncation of angiogenic domains in collagen alpha-1 (I). Moreover, SPCs retained specific proteins from the bovine serum supplement, including insulin-like growth factor-binding protein 2, a known target gene of the hypoxia-inducible factor, pigment epithelium-derived factor, a potent inhibitor of angiogenesis, and proteoglycans regulating collagen assembly. As a functional consequence, SPCs showed reduced invasive capacity and their conditioned medium inhibited endothelial tube formation. Conclusion. The present study represents an important conceptual development in vascular biology suggesting that SPCs can regulate tissue remodeling and demonstrates the utility of contemporary proteomics to better characterize vascular progenitors intended for therapy in clinics.
King's College London (2008) Proc Physiol Soc 13, C13
Oral Communications: Proteomic analysis reveals distinct role of smooth muscle progenitors in extracellular matrix production
D. Simper2,3, A. Didangelos1, U. Mayr1, C. Urbich4, A. Zampetaki1, M. Prokopi1, M. Mueller4, U. Benbow6, A. Newby6, R. Apweiler5, S. Rahman1, S. Dimmeler4, Q. Xu1, M. Mayr1
1. King's college London, London, United Kingdom. 2. Veterans Affairs Medical Center, Phoenix, Arizona, USA. 3. Arizona State University, Tempe, Arizona, USA. 4. Molecular Cardiology, Dept. of Internal Medicine,, Frankfurt, Germany. 5. EMBL/EBI, Hinxton, United Kingdom. 6. University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.