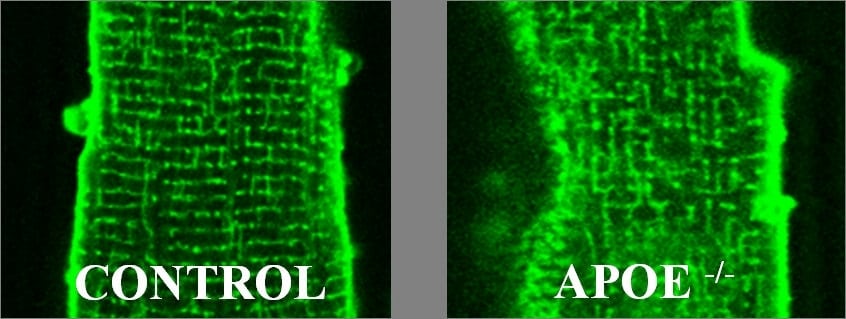
The apolipoprotein E knockout (apoE) mouse, when fed a high-fat diet, is an established model of atherosclerosis and coronary disease(1). Ventricular myocytes from the hearts of apoE mice show altered excitation-contraction coupling (ECC; this meeting). Since the t-tubule network plays a central role in ECC(2), and is disrupted in models of heart disease(3,4), we investigated whether t-tubular changes might contribute to the observed changes in ECC. Ventricular myocytes were isolated from the hearts of male apoE mice fed a high-fat diet for 6 months, and from wild-type (WT) mice fed a normal diet for 6 months. Intracellular Ca was recorded using fluo-3 during electrical stimulation (0.5 Hz) and during application of caffeine (20 mmol/L) to release Ca from the sarcoplasmic reticulum, in control and detubulated(5) WT and apoE myocytes. Figure 1 shows myocytes from WT and apoE mice, stained with di-8-ANEPPS. Compared with WT, apoE myocytes show a disorganised t-tubule structure. Detubulation decreased systolic Ca transient amplitude in WT and apoE myocytes, although the decrease was larger in WT myocytes (F/F0: 3.89±0.45 to 1.42±0.12 in WT; mean ± SEM; 63% decrease; p<0.0001; n=7/12 vs. 0.95±0.06 to 0.65±0.06 in apoE; 32% decrease; p=0.0052; n=9/6; unpaired t-test). Detubulation increased time to peak of the Ca transient to a similar extent in both groups (from 28±3 to 39±3 ms in WT; p=0.0431, and from 33±3 to 43±3 ms in apoE; p=0.0325), and slowed the decline of the Ca transient in WT, but not apoE, myocytes (rate constant (k): 10.92±0.16 to 7.75±0.86 s-1 in WT; p=0.040, and 6.15±0.34 to 6.11±0.31 s-1 in apoE; ns). Similarly, the decline of the caffeine-induced Ca transient was significantly slowed by detubulation in WT, but not apoE, myocytes (k: 0.53±0.04 to 0.33±0.04 s-1 in WT; p=0.0009; n=11/11, and 0.38±0.05 to 0.48±0.03 s-1 in apoE; ns; n=8/5). Thus time to peak of the systolic Ca transient, and the increase caused by detubulation, were similar in WT and apoE myocytes; this suggests that t-tubule remodelling in apoE myocytes has little effect on the Ca release process. In contrast, detubulation slowed the decline of the systolic and caffeine-induced Ca transients in WT but not apoE myocytes, suggesting decreased Ca extrusion across the t-tubule membrane in apoE cells. Such remodelling could result either from changes in t-tubule structure, or changes of protein expression and/or function.
University of Manchester (2010) Proc Physiol Soc 19, PC16
Poster Communications: T-tubule structure and function are altered in ventricular myocytes from apoE mice fed on a high-fat diet.
A. Chase1, C. H. Orchard1
1. Department of Physiology & Pharmacology, University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Figure 1.Representative sections of control and apoE myocytes stained with di-8-ANEPPS
Where applicable, experiments conform with Society ethical requirements.