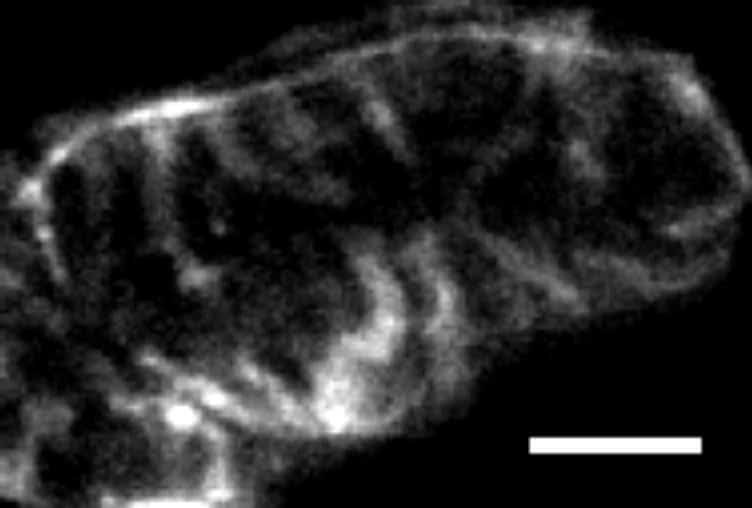
It is well known that the lateral resolution of a confocal microscope is superior to the axial resolution by a factor of 2-3 times. Thus cellular structure of the order of one micron in the axial plane cannot easily be resolved. Mammalian heart cells are ~120µm long at 20µm in width and depth. The poor axial resolution prevents structures in the cross section of the cell being resolved. To overcome this problem, microstructures were designed to hold a single heart cell in an “end-on” orientation in order to optimise conditions from imaging of the cross section. Templates for micro structures were created under clean-room conditions. Various structures of a proscribed height (e.g. 100µm) were made from SU-8 (Kayaka MicroChem, Japan) using photolithography. This template was used to create micro-bath units using Sylguard (184) (Dow-Corning) on the base of a coverslip. To arrange a isolated heart cells in a vertical position, the best structure was a series of wells, 100µm deep, and 100µm diameter (square section). Isolated rat and rabbit cardiac myocytes were produced by enzymatic dissociation of isolated Langendorff perfused hearts. Hearts were removed after cervical dislocation (Wistar rat) and a lethal i/v injection of 100 mg/kg Euthutal. (New Zeland White rabbit) Isolated myocytes were loaded into the microstructure using a quartz micropipette (tip diameter 40-60µm) from a separate adjacent bath. Vertical orientation was ensured by manipulating the cell against the vertical surface of the microsctructure. Cells remained upright due to limited attachment to the support structures. This system allowed vertical standing cardiomyocytes to be superfused with a range of solutions to allow labelling of several structures: (i) transverse-axial tubulular system (Figure 1) and the intercalated disc can be labelled using Di-4-ANEPPS (ii) mitochondria can be imaged using mitotracker dyes (iii) extracellular space can be imaged using Dextran labelled fluorescein. This technique reveals details concerning these structures that would otherwise have been unresolvable. Functional measurements (intracellular [Ca]) can also be made from cells orientated vertically to image radial details of Ca release.
University of Manchester (2010) Proc Physiol Soc 19, PC208
Poster Communications: Use of micro fabrication to create structures to support single heart cells at optimal orientation for axial imaging.
S. H. McIntyre1, N. Klauke2, J. Cooper2, G. Smith1
1. Integrative & Systems Biology, University of Glasgow, Glasgow, United Kingdom. 2. Electrical Engineering, University of Glasgow, Glasgow, United Kingdom.
View other abstracts by:
Figure 1. Confocal image of Di-4-ANEPPS stained rabbit ventricular myocyte orientated end on in a microfabricated structure. (Scale bar = 5 µm).
Where applicable, experiments conform with Society ethical requirements.