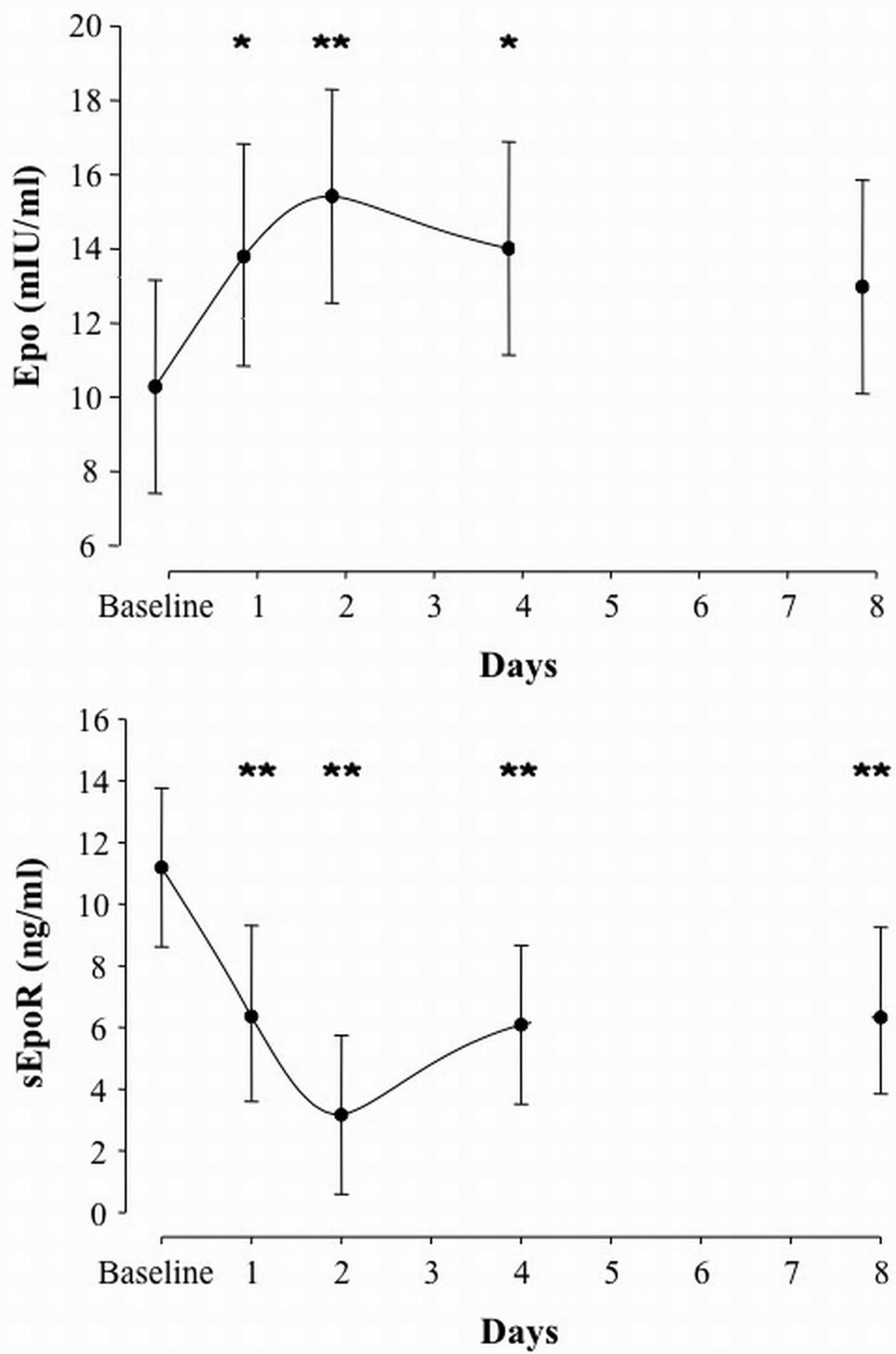
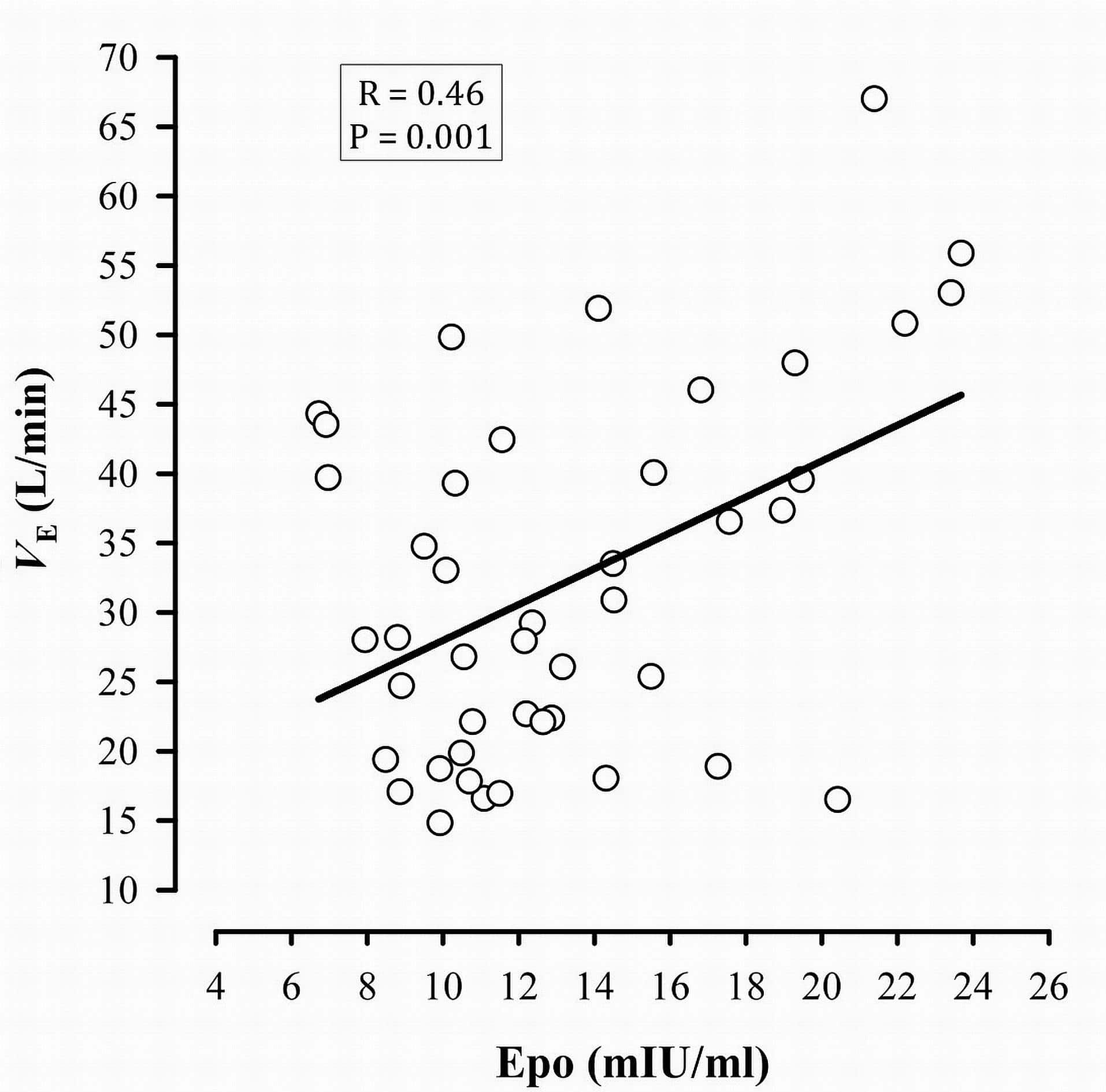
Upon exposure to acute hypoxia, the body responds almost instantly by an increase in ventilation due to the stimulation of the carotid bodies. A more efficient mechanism of adaptation, as experienced during chronic exposure to hypoxia, is an improvement in the oxygen carrying capacity following hypoxia-induced erythropoietin (Epo) secretion and accelerated erythropoiesis. During the last decade, extensive research on the mechanisms of action of Epo led to the discovery of potential new roles beyond the aforementioned hematopoietic effects. For instance, it has recently been demonstrated that Epo is involved in the ventilatory acclimatization to hypoxia in mice (Soliz et al., 2005). Subsequently, the same group also demonstrated this response is mediated by a decrease in soluble Epo receptors (sEpoR) (Soliz et al., 2007). We hypothesized that i) sEpoR would be downregulated by exposure to intermittent hypoxia (IHx), thereby allowing Epo concentration to rise; ii) these modifications would be correlated with the alteration in the acute hypoxic ventilatory response (AHVR) and breathing pattern (tidal volume – VT, breathing frequency – fR). Nine healthy male subjects (age: 29±5 yr, height: 173.8±9.5 cm and weight: 76.2±10.6 kg) were exposed to 6h of daytime IHx [2min normoxia (peak end-tidal PO2 (PETO2) = 88.0 Torr) and 2min hypoxia (nadir PETO2 = 45.0 Torr)] for four consecutive days (Days 1-4), preceded by two normoxic control days (4 days apart; combined as Baseline during analyses), and followed by one recovery day (4 days after the ned of IHx; Day 8). Epo and sEpoR concentrations and AHVR were measured on Baseline, Days 1, 2, 4 and 8. We observed a nadir in sEpoR on Day 2 (-70% vs. Baseline; p<0.01), concomitant with the peak in Epo secretion (+50% vs. Baseline; p<0.01) (Figure 1). Following each daily exposure to IHx, ventilation (VE) as measured during the AHVR test rose because of the increase in VT while fR remained unchanged. Similarly, AHVR increased progressively along with IHx (p=0.008). There was an overall negative correlation between Epo and sEpoR (r=-0.261, p=0.05), and between sEpoR and VT (r=-0.331, p=0.02). Epo was positively correlated with AHVR (r=0.475, p=0.001) and VE (r=0.458, p=0.001) (Figure 2). We conclude that sEpoR is downregulated upon exposure to IHx and thereby modulates the Epo response. Furthermore, the alterations in AHVR and breathing pattern following IHx seem at least partially mediated by the increase in Epo.
University of Birmingham (2010) Proc Physiol Soc 20, C12
Oral Communications: Effect of Intermittent Hypoxia on Erythropoietin, Soluble Erythropoietin Receptors and Ventilation in Healthy Humans
J. V. Brugniaux1,2, V. Pialoux2,7, G. E. Foster2,5, C. T. Duggan2, M. Eliasziw4, P. J. Hanly3,5, M. J. Poulin2,6
1. Neurovascular Research Laboratory, Faculty of Health, Sport & Science, University of Glamorgan, Pontypridd, United Kingdom. 2. Department of Physiology & Biophysics, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 3. Department of Medicine, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 4. Community Health Sciences, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 5. Hotchkiss Brain Institute, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 6. Libin Cardiovascular Institute of Alberta, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 7. Faculty of Kinesiology, University of Calgary, Calgary, Alberta, Canada.
View other abstracts by:
Figure 1. Serum Epo and plasma sEpoR concentrations over 4 days of intermittent hypoxia. * P ≤ 0.05 and ** P ≤ 0.01 vs. Baseline.
Figure 2. Correlation between Epo and minute ventilation (VE) measured during the AHVR test.
Where applicable, experiments conform with Society ethical requirements.