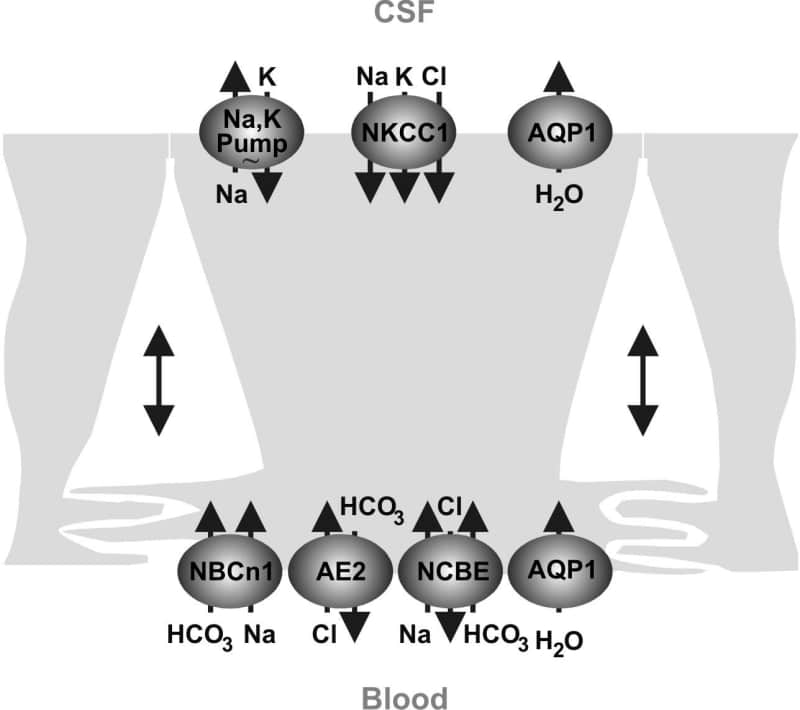
The choroid plexus epithelium secretes electrolytes and fluid into the brain ventricular system at high rates; comparable or exceeding the reabsorption rate of the renal proximal tubule. However, the mechanism by which the cerebrospinal fluid (CSF) is secreted is greatly different than in other fluid-secretory tissues e.g. the salivary or sweat glands. Studies of the mammalian choroid plexus suggest that the pivotal event in CSF secretion is the active transport of Na+ from the epithelial cell to the CSF, mediated by the apically positioned Na+,K+-ATPase (1). The apical Na+,K+,2Cl– cotransporter, NKCC1, may also contribute to the Na+ secretion and to recycling of K+ to the CSF (2) in concert with apical K+ channels (3). Cl–, however, is mainly secreted through electrogenic apical mechanisms, probably involving one or more Cl– channels (4). Water, in turn, would follow by a transcellular pathway, i.e. through the water channel AQP1 (5) or by a paracellular pathway. The Cl–/HCO3– exchanger, AE2, originally cloned from the rat choroid plexus (6), serves as a base extruder to maintain intracellular pH in many epithelia. AE2 may also contribute to basolateral Cl– uptake in the choroid plexus. The basolateral cellular entry mechanisms for Na+ and H2O into the choroid plexus are less well defined. The mRNA encoding the Na+/H+ exchanger, NHE1, is expressed in the choroid plexus of rat, and amiloride sensitive transport has also been detected several times by e.g. Conrad E. Johanson’s group. In addition, a basolateral DIDS sensitive Na+ and CO2/HCO3– dependent mechanisms seem to be involved in secretion in the presence of CO2/HCO3– (7, and others). Recently, it was suggested that the basolateral uptake of Na+ may be mediated by a Na+ dependent Cl–/HCO3– exchanger, NCBE. This transporter is expressed exclusively in the basolateral plasma membrane domain of epithelial cells in the choroid plexus epithelium and may explain the DIDS sensitive Na+:HCO3– cotransport in these cells. An electroneutral Na+:HCO3– cotransporter, NBCn1, is also expressed in the basolateral membrane of choroid plexus epithelial cells. NBCn1 is not likely to play a major role in the transepithelial movement of Na+ and HCO3– as it does not display the DIDS sensitivity of the main Na+ entry mechanism. The CSF secretion mechanism in humans is thought to be similar to the rodent system; however, few previous studies have addressed the molecular basis for choroid plexus transport in humans. Therefore, we aimed to localize several of the aforementioned proteins to the human choroid plexus. The Na+,K+-ATPase α1-subunit is localized apically in the human choroid plexus epithelial cells, as was NKCC1. AQP1 is predominantly situated in the apical plasma membrane domain, although weaker basolateral immunoreactivity is also observed. AE2 is localized basolaterally, as is NCBE and NBCn1. No NHE1 immunoreactivity is found. Hence, the human choroid plexus epithelium displays an almost identical distribution pattern of water channels and Na+ transporters as the rat and mouse choroid plexuses (Fig. 1). In conclusion, it remains to be determined how Na+ enters the choroid plexus epithelium, how Cl– and HCO3– exit the cells. Moreover, precise estimates of the ionic gradients and subcellular localization of the involved transporters are warranted to better model the CSF secretory process.
University of Manchester (2006) Proc Physiol Soc 2, SA12
Research Symposium: Distribution of ion transporters in the choroid plexus – an atypical epithelium
Jeppe Praetorius1
1. Institute of Anatomy, The Water and Salt Research Center, University of Aarhus, Aarhus, Denmark.
View other abstracts by:
Figure 1. Model showing the localisation of the Na+ K+-ATPase AQP1 NKCC1 and HCO3- transporters in the human choroid plexus based on the present and previous observations. The water channel AQP4 the Na+/H+ exchanger NHE1 and the electrogenic Na+-base cotransporters NBCe1 and NBCe2 were not detected in the human choroid plexus.
Where applicable, experiments conform with Society ethical requirements.