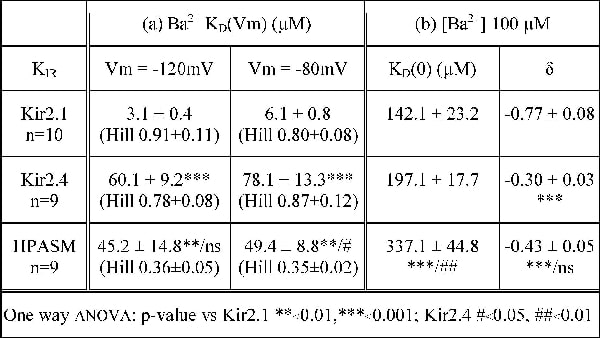
Strong inward rectifier K+ currents (KIR), carried by Kir2.0 channels, are thought to contribute to the resting membrane potential in smooth muscle, predominantly through Kir2.1 (1). Kir2.0 currents differ in their sensitivity to barium inhibition and all, except Kir2.4, show a characteristic voltage and time-dependence to the block (2). We electrophysiologically characterised the Ba2+ inhibition of KIR currents, comparing native human pulmonary arterial smooth muscle (HPASM) cell currents with cloned Kir2.1 or Kir2.4. Whole-cell currents were recorded from HPASM cells and HEK293 cells, stably transfected with Kir2.1 or Kir2.4, in the presence of a 60/140mM K+ gradient. At a holding potential of -20mV, voltage steps were applied from -150mV to +20mV and extracellular Ba2+ block of current was examined (0.1µM-10mM). The concentration dependency of barium block was assessed through inhibition curves at various membrane potentials (Table 1a). Interestingly, the Hill coefficient for KIR in HPASM cells was shallow (~0.4), which may represent a contribution from at least two distinct molecular components to the current. Kir2.1 currents showed greater sensitivity to Ba2+ compared with those of Kir2.4 and HPASM cells at -120mV. Currents from all three cells showed distinct Ba2+ sensitivities at -80mV, with HPASM cells being intermediate. Kir2.1, additionally showed a 2-fold decrease in Ba2+ sensitivity when depolarised from -120 to -80mV (p<0.01) whereas Kir2.4 and HPASM cells showed no significant decrease (t test). The voltage dependence of Ba2+ block of KIR was assessed quantitatively using the approach of Woodhull (3), the parameters of which are shown in Table 1(b).The fractional distance of Ba2+ block into the transmembrane field, δ, for Kir2.1 was distinct from that of Kir2.4 and KIR in HPASM cells, which themselves were indistinct. The KD(0) value reflects the sensitivity to Ba2+ block at 0mV and thus removes any contribution of voltage dependence. Currents in HPASM cells were different to both Kir2.1 and Kir2.4 currents, but Kir2.1 and Kir2.4 were not distinct. Together these data suggest that the molecular components of KIR currents in HPASM cells are not solely composed of Kir2.1. The characteristics described here, indicate that more than one Kir2.0 channel may contribute and that Ba2+ block of HPASM cell KIR shares more similarity to Kir2.4 than Kir2.1.
University College London 2006 (2006) Proc Physiol Soc 3, PC35
Poster Communications: Barium block of inward rectifier potassium channels: comparison of cultured human pulmonary smooth muscle cells with cloned Kir2.1 and 2.4
Brian P Tennant1, Yi Cui1, Andrew Tinker1, Lucie H Clapp1
1. Medicine, University College London, London, United Kingdom.
View other abstracts by:
Table 1
Where applicable, experiments conform with Society ethical requirements.