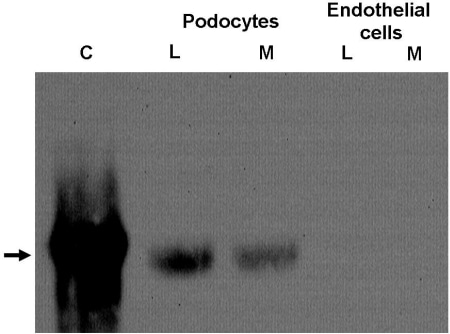
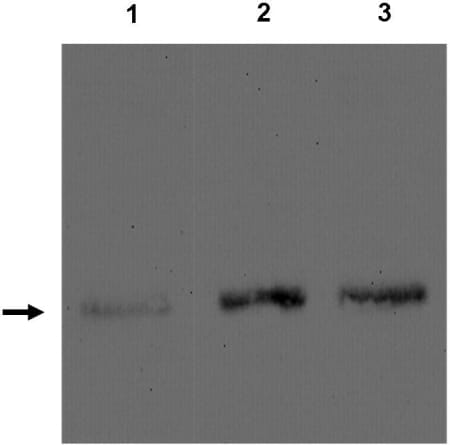
In diabetic nephropathy, the most important cause of renal failure in the developed world, overt albuminuria is a marker for a poor prognosis not only from renal disease itself but also from associated cardiovascular disease[1-3]. How systemic disorders of insulin action lead to detrimental effects on the glomerular filtration barrier GFB is surprisingly poorly understood. There is accumulating evidence that the podocyte is central to the development of diabetic nephropathy, although the mechanism is not known[4]. We have recently reported the novel observation that human podocytes are insulin sensitive cells[5]. It is now recognised that adipocyte derived factors are important in regulating insulin sensitivity of distant tissues. The levels of one such factor, adiponectin, have been shown to be altered in end stage renal disease This laboratory has developed and characterised in detail human conditionally immortalised podocyte and endothelial cell lines[6,7] We have been studying the effect of adiponectin on these cell lines. Using RTPCR and western blotting techniques we have found that podocytes, but not endothelial cells, produce adiponectin and secrete it into the media (Fig 1). This is suprising since this factor was thought to be solely produced by adipocytes. Like adipocytes, adiponectin production is regulated by insulin (Fig 2.). Immunoflurescence analysis demonstrates that the adiponectin is localized within the podocyte to intracellular vesicles. Both cell types express adiponectin receptors suggesting that they both respond to adiponectin. Using phosphospecific antibodies we are at present looking at the activation of various cell signalling pathways known to be activated in other cell types by adiponectin namely the Protein Kinase B, MAPK and AMPK pathways. Conclusion: Our results, demonstrating surprisingly that podocytes produce and secrete adiponectin, a key regulator of insulin sensitivity and tissue inflammation [8], point to potential new mechanisms in the regulation of the physiology of the cells of the glomerular filtration barrier. Furthermore these results may point to a novel component in the pathogenetic mechanism of early diabetic nephropathy.
University of Bristol (2008) Proc Physiol Soc 9, C11
Oral Communications: A novel role for adiponectin in the glomerular filtration barrier?
F. Swan1, J. Fabian1, J. P. Shield1, P. W. Mathieson1, M. A. Saleem1, G. I. Welsh1
1. Academic Renal Unit, University of Bristol, Bristol, United Kingdom.
View other abstracts by:
Fig.1 Analysis of adiponectin levels by Western blot of cell lysates (L) and culture media (M) from glomerular podocytes and endothelial cells. Position of adiponectin shown by arrow. C corresponds to a lane run using recombinant adiponectin as a control.
Fig 2. Analysis of adiponectin levels byWestern blot of podocyte cell lysates grown in the presence (1) or absence of insulin for 24 (2) or 48 hours (3). Position of adiponectin shown by an arrow.
Where applicable, experiments conform with Society ethical requirements.