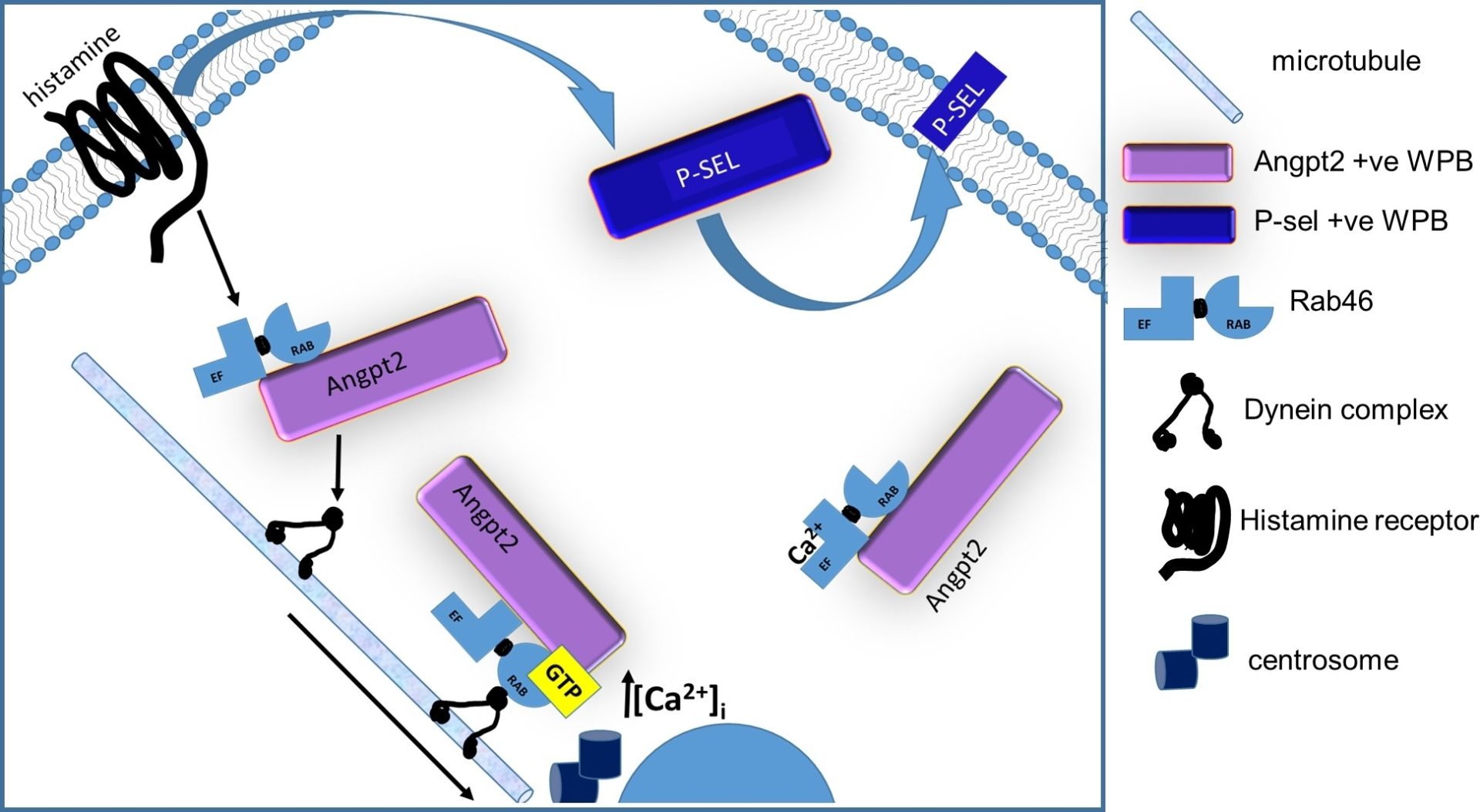
The release of pro-coagulant and pro-inflammatory mediators stored in endothelial-specific vesicles (Weibel-Palade bodies: WPBs) is necessary for an efficient and rapid response to vascular injury. This emergency response to vascular injury is vital but potentially risky because, in addition to injury, there are other, functionally distinct, stimulants that promote WPB exocytosis. The raised levels of WPB cargo in the serum of patients with cardiovascular diseases suggest the inappropriate and untimely exocytosis of WPBs can promote the pathological thrombotic environment necessary for disease development. Immunogenic stimuli evoke WPB exocytosis resulting in an immune response without inducing coagulation or wound healing. Whilst the mobilisation of intracellular Ca2+ is necessary for WPB exocytosis, the mechanisms underlying the differential release of cargo in order to produce these physiologically distinct responses are poorly understood. Recently, we described a novel Rab GTPase (Rab46: CRACR2A-L; CRACR2A-a) in endothelial cells that has GTPase and Ca2+ binding activities and is localized to WPBs. To explore the role of Rab46 in endothelial cells we utilized high-resolution AiryScan imaging alongside calcium imaging and biochemical techniques. We demonstrate that Rab46 is localised to a subset of WPBs (49% +/- 13%). Acute stimulation of endothelial cells with the pro-inflammatory mediator histamine (but not the pro-thrombotic agonist thrombin), evoked trafficking of a subpopulation of WPBs to the microtubule organising centre (MTOC). This retrograde trafficking is dependent on the nucleotide-bound form of Rab46 and dynein-mediated transport of WPBs along microtubules. MTOC localised WPBs are devoid of P-selectin, the adhesion receptor for leukocytes, but store cargo superfluous to an inflammatory response, for example angiopoietin-2. Thus, whilst histamine stimulates release of P-selectin from WPBs at the cell surface thereby supporting an inflammatory reaction, histamine limits an excessive vascular injury response by anchoring WPBs containing cargo extraneous to inflammation to the MTOC. Dissociation of the perinuclear localised WPBs requires a continuous mobilisation of intracellular Ca2+ evoking binding of Ca2+ to the EF-hand of Rab46. In this way, Rab46 integrates transport and Ca2+ signals to regulate trafficking of WPBs that is relevant to the physiological signal and thus limits the extensive emergency response evoked by vascular injury. We propose that understanding the molecular mechanisms underlying Rab46 function will provide targets for therapeutic intervention of cardiovascular disease.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, C112
Oral Communications: A Ca2+-regulated G protein (Rab46) couples inflammatory stimuli to differential trafficking of Weibel-Palade bodies
K. Miteva1, L. Pedicini1, D. Beech1, L. McKeown1
1. LICAMM, University of Leeds, Leeds, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.