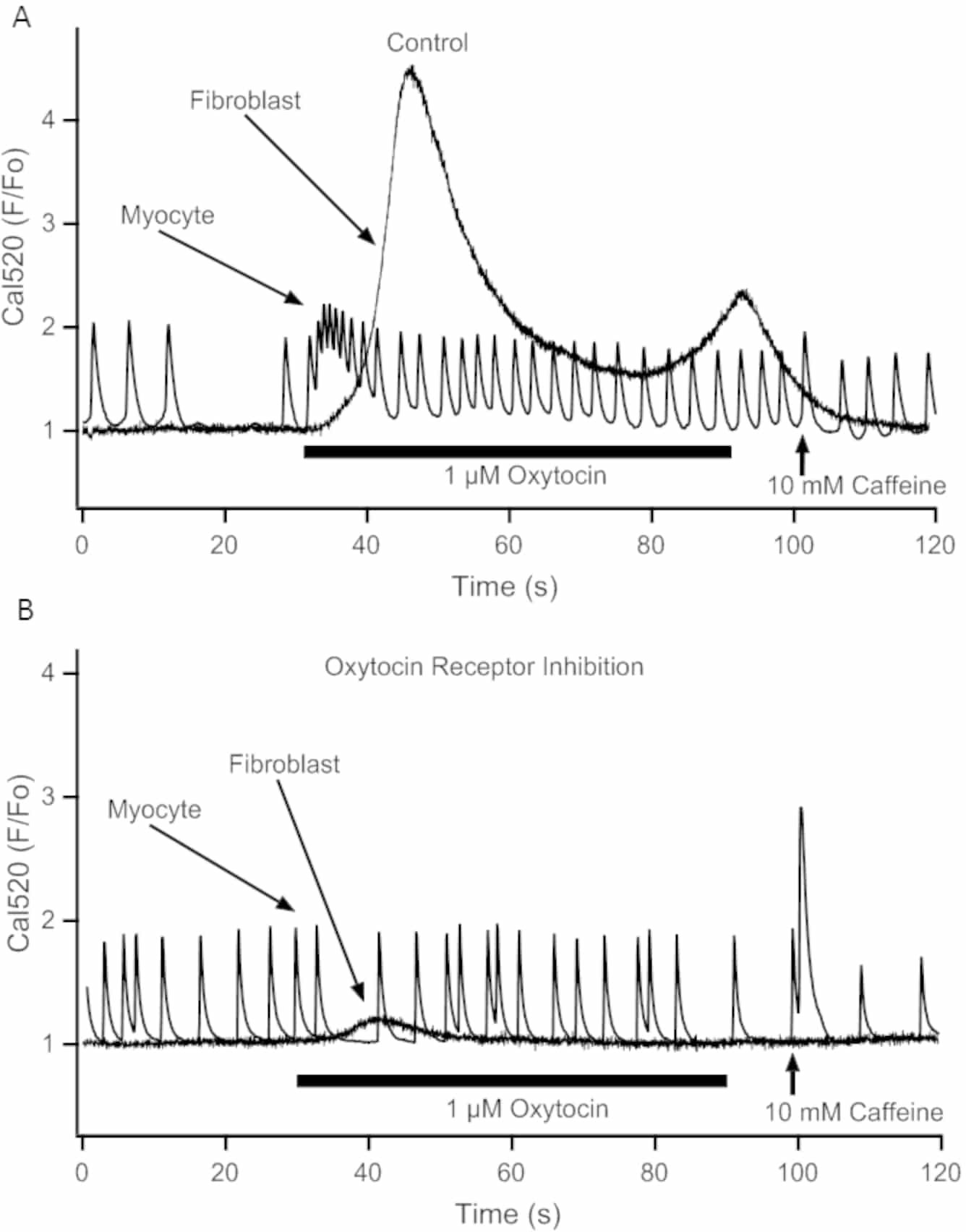
Oxytocin receptors are expressed in mammalian heart. Systemic injections of oxytocin cause negative chrono- and inotropic effects that were linked to the release of atrial natriuretic peptide [1]. There is a paucity of data describing direct effects of oxytocin on intracellular calcium ([Ca2+]i) in cardiac myocytes and a complete lack of data in cardiac fibroblasts. Our aim was to investigate the acute responses of cardiac myocytes and cardiac fibroblasts to the application of this neuropeptide. Cells were enzymatically isolated from 1 day old Wistar rat pups and maintained in short-term (up to four days) primary culture. The use of neonatal rats for this study has been approved by the UAEU Animal Ethics Committee (ERA_2017_5681). Spontaneous intracellular Ca2+ transients were recorded using the Ca2+ sensitive fluorescent dye Cal520 and a fast EM-CCD camera. Sequences of fluorescence images were taken at a rate of 40 Hz. Oxytocin was applied for 60 s after a 30s long baseline recording. The recording continued for an additional 30 s after oxytocin washout. During this time interval, 10 mM caffeine was applied to distinguish the cardiac myocytes from the fibroblasts as the latter cell type does not respond to caffeine. Image acquisition and quantification were performed using the WinFluor software package (J. Dempster, Strathclyde University). Mean fluorescence was measured from ROIs placed over all cells in the entire field of view and presented as self-ratio (F/F0). Where appropriate, statistical comparisons were made using paired t-test or 1-way ANOVA followed by Dunnet’s post-hoc test in IgorPro 7.0 (Wavemetrics, USA). Within 24 hours, cardiac myocytes developed spontaneous contractions accompanied by transient rises in [Ca2+]i while cardiac fibroblasts remained quiescent and maintained low [Ca2+]i. Application of 1 µM oxytocin to cardiac myocytes caused an increase in the frequency of [Ca2+]i transients from 0.13 ± 0.01 to 0.29 ± 0.03 Hz and decreased their amplitude from 2.25 ± 0.1 to 1.73 ± 0.08 F/F0 (n=21, p <0.01, paired t-test). In the fibroblasts, 1µM oxytocin induced large [Ca2+]i transients averaging 3.59 ± 1.08 F/F0 ( illustrated in Fig.1A). The observed effects were abolished in all cells after pre-incubation with selective oxytocin receptor inhibitor L-371.257 (10mM) suggesting that they were mediated by activation of the oxytocin receptors and were not due to the cross-activation of vasopressin receptors that are also expressed in the heart (Fig.1B). Oxytocin induced [Ca2+]i transients in cardiac fibroblasts were abolished by a non-selective IP3 receptor inhibitor 2-APB and disappeared in the presence of the SERCA pump inhibitor thapsigargin. In conclusion, oxytocin exerts direct positive chronotropic and negative inotropic effects on the neonatal cardiomyocytes and induces IP3 mediated Ca2+ release in cardiac fibroblasts.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, PC011
Poster Communications: Effects of oxytocin on Ca2+ signalling in newborn rat cardiac myocytes and cardiac fibroblasts.
I. I. Ali1, S. Al-Salam2, F. C. Howarth1, A. Shmygol1
1. Department of Physiology, United Arab Emirates University, Al Ain, United Arab Emirates. 2. Department of Pathology, United Arab Emirates University, Al Ain, United Arab Emirates.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.