
Physiology News Magazine
Hold your nerve
Is the nervous system to blame for muscle loss in old age?
Features
Hold your nerve
Is the nervous system to blame for muscle loss in old age?
Features
Jamie McPhee, Manchester Metropolitan University, Manchester, UK
https://doi.org/10.36866/pn.111.32
Decreased skeletal muscle mass and reduced physical function in older age, commonly known as sarcopenia, is a serious condition affecting 10–20% of people aged over 65 years and is projected to affect 20–30 million Europeans by 2045. In recent years, our aim has been to develop a better understanding of the neuromuscular changes that contribute to sarcopenia. So, what have we learned?
General physical function
Let’s begin by stating the obvious – on average, older people move around more slowly than younger people and the older a person becomes, the slower they move. This slowness is observed in many types of everyday activities such as sit-to-stand transitions and ascending or descending stairs, but is best represented by walking speed. A typical young adult with a walking speed of 1.85 ms-1 (measured during a six-minute walking test) would complete a mile in just under 15 minutes, which is 3 minutes faster than a typical 75-year-old walking at 1.49 ms-1. Not so much difference between young and old, you may think, but there is considerable inter-individual variability between people of the same age. Slowness is a feature of physical frailty affecting about half of people aged over 80 years (typically walking 0.8 ms-1). Such slow walking speeds are associated with a higher risk of mortality. Indeed, it could be said that the Grim Reaper’s preferred walking speed is 0.82 ms-1; a quirky conclusion reached by Stanaway et al. (2011) based on the hazard ratio of all-cause mortality being highest for people walking at this speed or slower. These are, of course, just associations, and it is unlikely that walking slowly is a direct cause of death, in the same way that grey hair is associated with ageing and infirmity but not its cause.
Nevertheless, loss of mobility has a major impact on quality of life. Not only does it reduce a person’s ability to look after themselves, but slow and hesitant movements also increase susceptibility to trips and falls that can have life-changing consequences. The decline in physical function is due, at least in part, to older adults having smaller, weaker muscles than younger adults. Hand-grip strength is commonly used to demonstrate this. Perhaps unsurprisingly, low grip strength in older age (typically <30 kg for men and <20 kg for women) is associated with an increased risk of all-cause mortality. Presumably, this association has nothing to do with one’s ability to wrestle off the Grim Reaper, but is instead a component of what geriatricians have termed ‘robustness’.
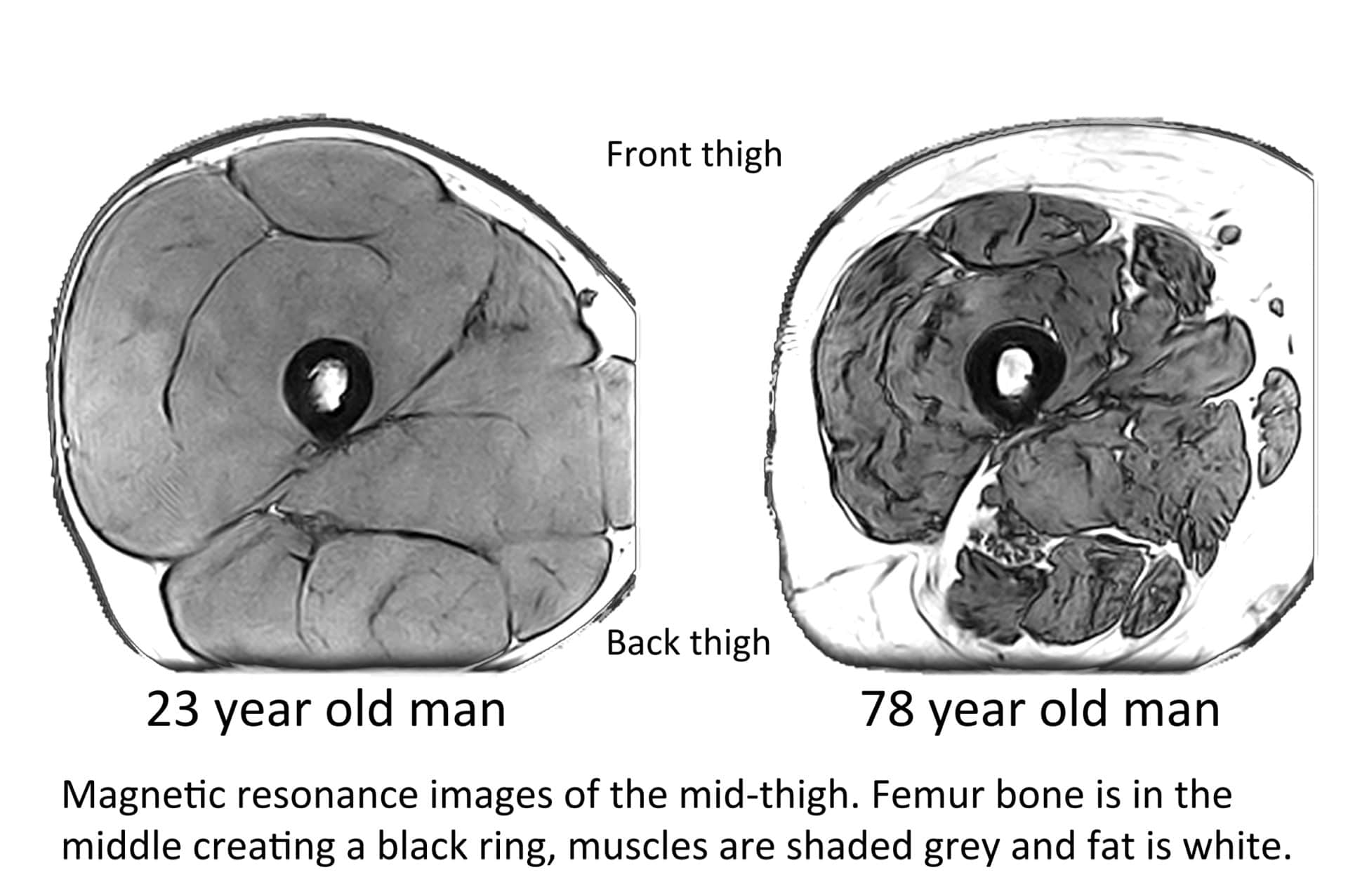
Large epidemiological studies tend not to include leg strength measurements, and this is unfortunate because physiological studies, which are generally smaller in scale but more detailed in methods than epidemiological studies, show that muscle changes with ageing are not straightforward processes. Muscles are not all equally susceptible. Indeed, grip strength is not representative of the performance of all muscles. When measured by magnetic resonance imaging (MRI), whole-body muscle mass in 75-year-olds was on average 15% lower when compared to 25-year-olds, but a closer look revealed upper body muscle mass was just 10% lower while leg muscle mass was almost 25% lower in older versus younger study participants (Janssen et al., 2000). The large quadriceps muscles of the front thigh seem particularly susceptible to wasting and are typically about 33% smaller in 75-year-olds than 25-year-olds (McPhee et al., 2018) (Fig. 1).
Measurements of maximal strength follow the same pattern of upper-limb versus lower-limb discordance observed in muscle mass. Knee extensor strength is typically around 40% lower in 75-year-olds compared to 25-year-olds, which is much greater than the 25% lower grip strength observed.
There is no question that thigh muscle weakness reduces maximal running speed and increases the effort required to negotiate stairs, but is it the cause of the decrease in walking speed and general mobility? After all, walking is not a maximal effort activity. Some studies addressing this question by pooling data from a wide range of ages and health states found that stronger people walked faster. However, within a given age group (be it younger or older) and after separating the sexes the relationship is in fact relatively weak. This is clearly demonstrated by older men having very similar strength to younger women, yet younger women walk faster and are more agile than older men. Of course, changing cardiopulmonary fitness and age-related decline of maximal heart rate, as well as orthopaedic and rheumatic conditions, a general awareness of vulnerability due to poor balance, and reduced ability to dual-task must also be taken into account.
Muscle quality and quantity
Keen readers will have noted that loss of strength is greater than the loss of muscle mass in older age. This is indeed the case.
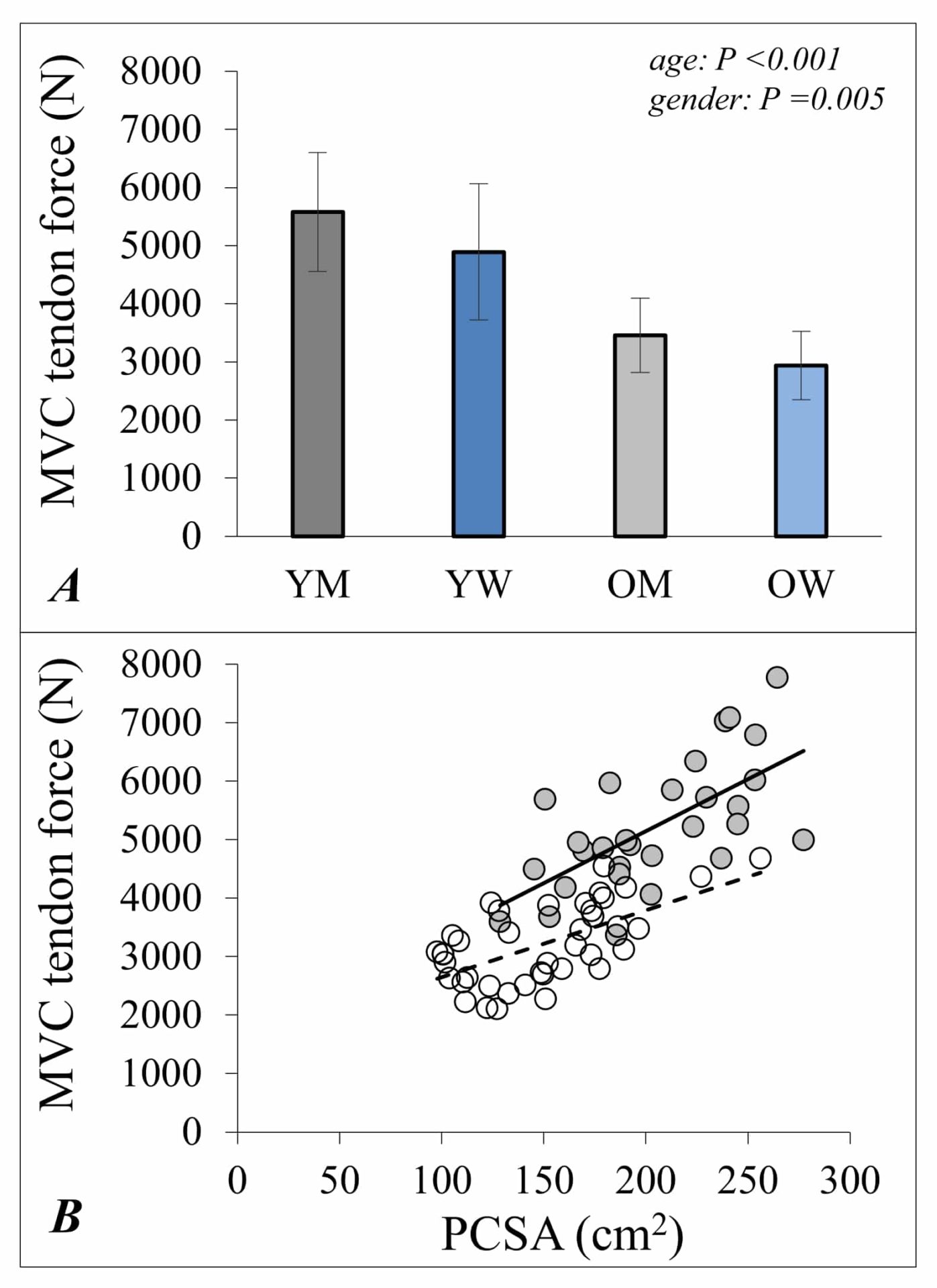
While overall it remains true that larger muscles (determined by greater cross-sectional areas with more sarcomeres in parallel) are stronger muscles, the force produced per unit muscle mass is lower for older than it is for younger adults (McPhee et al., 2018) (Figure 2).
A great deal of effort has gone into understanding why muscle “quality” declines in this way. It is important to remember that strength, the outward expression of muscle activity, is not only determined by the sum of forces produced from individual muscle fibres. The measured maximal strength also depends on all available motor units (the combination of alpha motor neurons and innervated muscle fibres) of the agonist muscles being fully activated and the architecture of muscle and joint structures. These features of muscle architecture and motor unit activation also change as part of the ageing process and, after accounting for these changes, it is possible to estimate the “muscle quality” as strength per unit muscle mass (known as “in situ specific force”). A recent study showed that this measurement of muscle quality was reduced by 17% in older adults’ quadriceps compared with younger adults (McPhee et al., 2018) and this corresponds closely with a 16% lower specific tension reported for single muscle fibres (Brocca et al., 2017). A 5-year follow-up of the older participants revealed accelerated declines in muscle mass and strength through the eighth decade compared with estimates of changes made from age 30-70 years (McPhee et al., 2018).
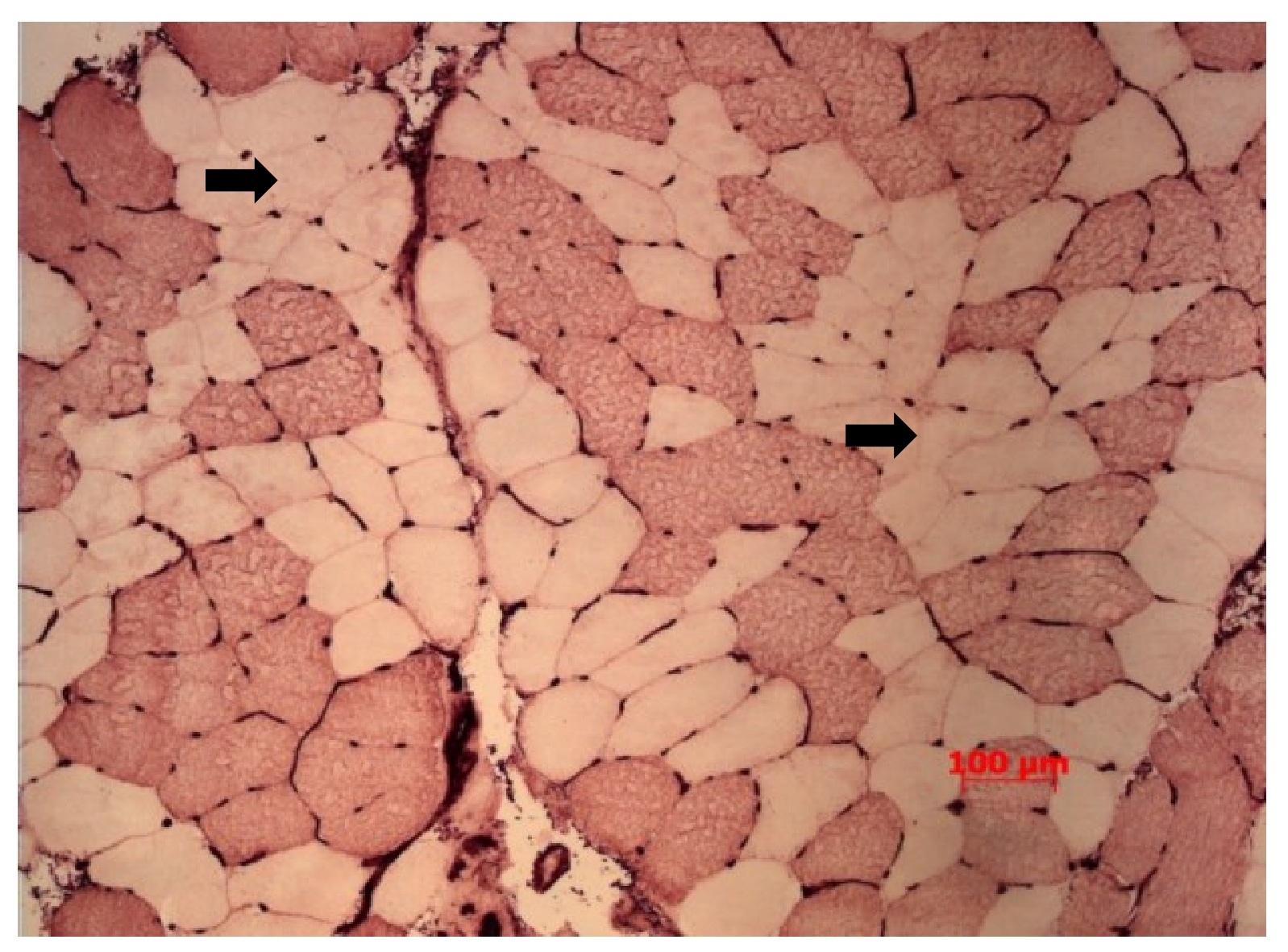
Although reduced muscle ‘quality’ and motor unit activation contribute to weakness in older age, they explain only about a third of the reduced strength. The remaining two-thirds is due to low muscle mass. Muscle mass is lost with ageing for two main reasons. First, there is a reduction of the average muscle fibre cross sectional area, particularly affecting the larger and more powerful type 2 fibres. On average, these account for approximately 55% of total vastus lateralis (one of the four quadricep muscles) muscle fibre area in younger people and they atrophy by around 25% in older age (McPhee et al., 2018). Muscle fibres of older people also show increased variability of size with some small, angular fibres and some enclosed fibres, which are hallmarks of cycles of denervation-reinnervation (Figure 3).
The second cause of muscle loss in older age is reduction of muscle fibre numbers. However, there is surprisingly little information available about how many fibres there are in human muscles. We estimate that fibre atrophy accounts for 54% of the difference between younger and older vastus lateralis muscles, with the remaining 46% due to approximately 18% of the muscle fibres being lost (McPhee et al., 2018). Type 1 and type 2 fibres are both lost during ageing.
Motor units
The loss of muscle mass in older age may be linked to declining numbers of motor units.
A motor unit comprises a single alpha motor neuron and the muscle fibres it innervates. The motor neuron cell body is located in the ventral horn of the spinal cord and projects an axon towards a target muscle where it branches to innervate muscle fibres. Large limb muscles are composed of thousands of motor units, each innervating thousands of muscle fibres. If a motor neuron dies or the axon is damaged during ageing, associated muscle fibres may lose their innervation status and become atrophied and vulnerable to apoptosis.
Direct counts of neuron cell bodies from autopsy samples in the 1970s showed declining numbers of motor neurons from age 60 years. More recent electrophysiological estimates of the quadriceps (Fig. 4) and tibialis anterior muscles suggest that 70-year-old men have on average 40%fewer motor units compared with younger men (women were not assessed). Motor unit loss appears to exceed the loss of muscle mass and precedes sarcopenia. This is because of a compensatory mechanism whereby branching of nearby healthy motor axons can reinnervate orphaned fibres, effectively rescuing them from degradation (Piasecki et al., 2018).
We do not yet know why motor neurons are lost with advancing age, nor do we know why some, but not all, orphaned muscle fibres are reinnervated. These are important areas of future research because evidence suggests that motor neurons and muscle fibres cannot be recovered once lost. It is appealing to think that motor units may be preserved in older age if they are frequently used for muscle contractions, leading to the idea that regular exercise training may protect against the full extent of motor unit loss. We have looked into this hypothesis and found no evidence that athletic older people who had been training and competing for endurance or sprint running events most of their adult lives were spared from motor unit losses. Unfortunately, we know of no way to prevent, reverse or even slow this process.
The benefits of regular exercise
If regular training does little to preserve motor unit numbers, what, then, are the benefits of remaining physically active into older age?
Well, the more active of you will be pleased to know that the benefits are plentiful. Anyone attending master athlete competitions will know of their dedication to training and the remarkable athletic performances of people into their 80s and, in some cases, 90s or even beyond. For instance, some 75-year-old British men run 5000m in less than 20 mins, which is almost 10 mins faster than the typical time posted by a keen middle-aged park runner on a Saturday morning. Notwithstanding the achievements of exceptionally dedicated older athletes lucky enough to avoid limiting musculoskeletal complaints, there is still plenty of good news for the average older person because muscles and other body systems remain highly responsive to exercise training.
Endurance training performed at reasonably high intensity will increase cardiopulmonary fitness and muscle metabolism (McPhee et al., 2016), helping to dispose of fatty acids and glucose and to utilise, rather than accumulate, fat stores. However, few older people are willing to take up running or other intense endurance activities. Instead, it is far more common for middle-aged and older people to complete relatively low intensity activities such as walking. Lower intensity activities are great for breaking up prolonged periods of sitting and can have many benefits for social engagement and wellbeing, but they are unlikely to improve muscle mass, strength, or balance; thus, they may not prevent muscle weakness or falls risks in older age.
A resistance training programme can help to ameliorate type II fibre atrophy and improve muscle specific force (McPhee et al., 2016). There are also adaptations within the connective tissue and tendons that make up the functional muscle tendon unit and adapted patterns of motor unit recruitment for better coordination of movements. These adaptations could increase walking speed and general physical function.
In the absence of effective or acceptable pharmacological and nutritional interventions, resistance training remains the best available intervention to combat sarcopenia. However, few older people are fond of lifting weights in the local gym. Perhaps there are cultural or confidence barriers, or perhaps it is inconceivable for healthcare providers who do not themselves lift weights in the local gym to prescribe it for older people to combat weakness and low muscle mass.
For these reasons, mixed exercise sessions involving some light resistance, moderate intensity endurance, and balance exercises are recommended to maintain or improve physical function amongst older people. Yet, the largest trial of mixed exercise training for previously sedentary older adults at risk of developing weakness showed very little improvement of physical function after training three times per week for more than 24 months (results of the LIFE Study, e.g. see Santanasto et al., 2017). This might indicate diminishing returns in older age, possibly due to irreversible motor unit declines that already occurred, or it could be that the resistance exercise allocation was simply not enough.
Either way, there is no easy answer to the question of what types of exercise older people should do to maintain health and physical function. For now, the pragmatic response is that everyone should complete activities they enjoy because they are more likely to maintain these activities over the months and years.
Acknowledgements
The research described here was funded by the European Commission (FP7 “MYOAGE” 223576) and the Medical Research Council (MR/K/025252/1). I am very grateful for the vibrant and enthusiastic team of researchers involved in the studies described here (named in the references list) and to the participants for their willingness to get involved in research.
References
Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M & Bottinelli R. (2017). Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595, 4823-4844.
Janssen I, Heymsfield SB, Wang ZM & Ross R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 89, 81-88.
McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, Jones DA & Degens H. (2018). The contributions of fibre atrophy, fibre loss, in situ specific force and voluntary activation to weakness in sarcopenia. J Gerontol A Biol Sci Med Sci.
McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N & Degens H. (2016). Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 17, 567-580.
Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA & McPhee JS. (2018). Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol 596, 1627-1637.
Santanasto AJ, Glynn NW, Lovato LC, Blair SN, Fielding RA, Gill TM, Guralnik JM, Hsu FC, King AC, Strotmeyer ES, Manini TM, Marsh AP, McDermott MM, Goodpaster BH, Pahor M, Newman AB & Group LS. (2017). Effect of Physical Activity versus Health Education on Physical Function, Grip Strength and Mobility. J Am Geriatr Soc 65, 1427-1433.
Stanaway FF, Gnjidic D, Blyth FM, Le Couteur DG, Naganathan V, Waite L, Seibel MJ, Handelsman DJ, Sambrook PN & Cumming RG. (2011). How fast does the Grim Reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ 343, d7679.
