
Physiology News Magazine
Early to bed and early to rise
Makes a teen healthy, wealthy and wise?
Features
Early to bed and early to rise
Makes a teen healthy, wealthy and wise?
Features
Gaby Illingworth
Rachel Sharman
Russell Foster
Sleep and Circadian Neuroscience Institute, Nuffield Department of Clinical Neurosciences, University of Oxford, UK
https://doi.org/10.36866/pn.110.22
In adapting this well-known saying to suit our purposes, we haven’t entirely used poetic licence. How many times have you heard people commenting that, given the option, their teenage offspring routinely stay up late at night and then have a long lie-in the next day? Sometimes this is put down to laziness or an individual’s transition from childhood into ‘Kevin the Teenager’. However, there are biological reasons which help explain why teenagers have a tendency to be late to bed and late to rise, and why they might not actually be getting enough sleep to make them healthy, wealthy and wise.



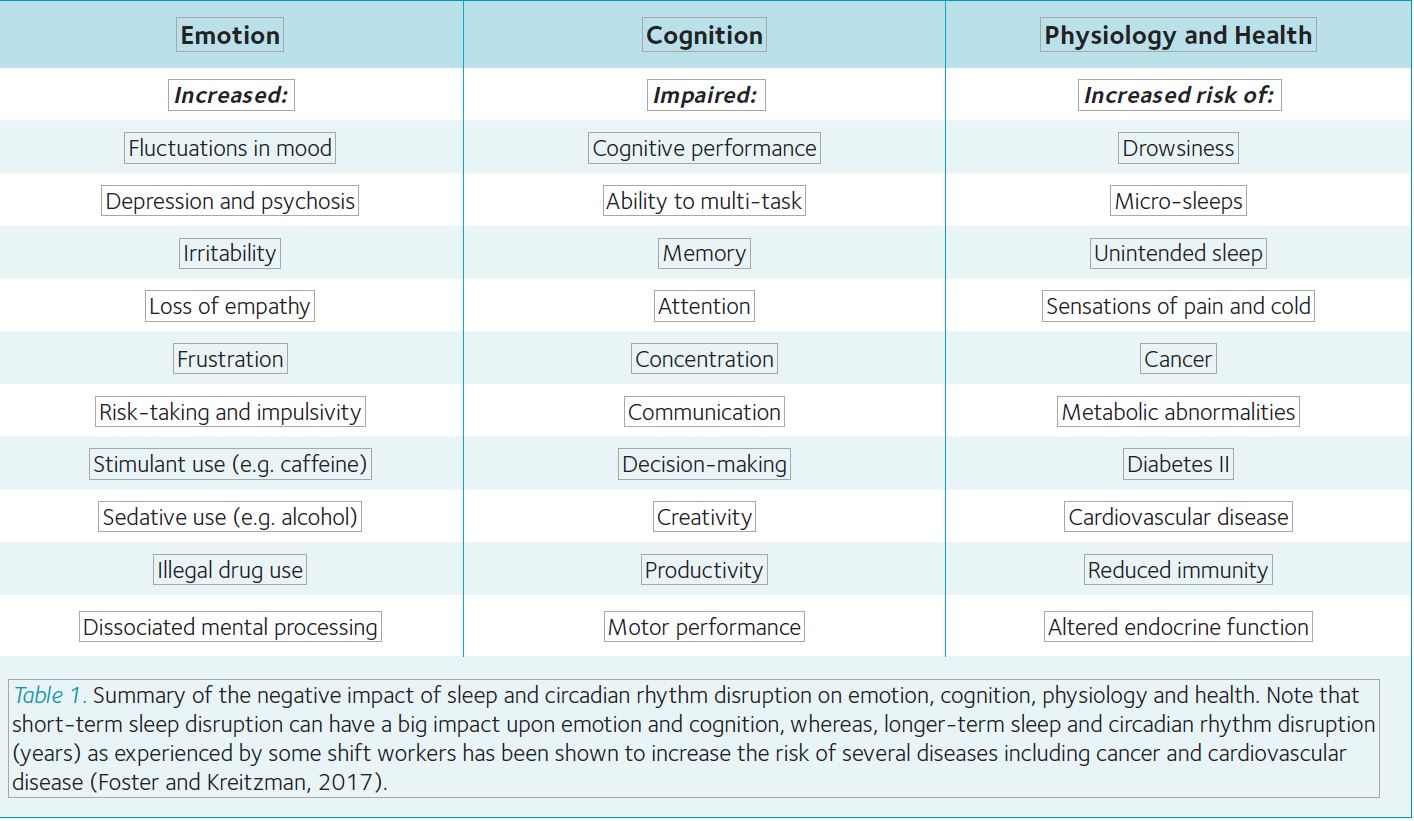
Sleep matters. A wealth of evidence supports the importance of sleep for our physical, cognitive and emotional functioning. For example, insufficient sleep has been linked with impairments in attention, creativity, memory consolidation and academic performance. It has also been associated with behaviour problems, increased impulsivity and difficulties with mood regulation (Table 1). We are therefore interested in the characteristics of teenage sleep, and how sleep during this key stage of human development may be improved.
The biology of sleep
In order to understand the bioregulation of teenage sleep patterns, you first need to know about the drivers of sleep. Borbély proposed the two-process model of sleep in 1982, which has since become the most widely used model. Sleep is explained as driven by two processes working in tandem: the circadian rhythm and the sleep/wake homeostat. A circadian rhythm is a roughly 24-hour cycle of internally-generated oscillations in physiology and behaviour. These endogenous rhythms are generated through the activation and suppression of clock genes within almost all cells throughout the body creating a cellular timing network. The ‘master biological clock’, the suprachiasmatic nucleus (SCN), situated in the hypothalamus, coordinates these rhythms within the peripheral cellular clocks; this network is collectively known as the ‘circadian system’.
The timing of sleep and wakefulness is perhaps one of the most easily observed circadian behaviours. ‘Chronotype’ refers to our preferred sleep/wake timing, demonstrated by the time of day when you feel most sleepy or alert. Small changes in clock genes have been linked to a genetic basis for chronotype. A biological clock (or internal circadian period) that runs faster would mean that, left to your own devices, you would go to sleep and wake up early, colloquially known as being a ‘lark’. On the other hand, a slower clock will drive the sleep/wake cycle to a later time and you would be an ‘owl’.
The endogenous period of our internal clock isn’t exactly 24 hours so needs to be adjusted daily through zeitgebers, or time-givers, with changes in light intensity at dawn and dusk being the principal entraining factor. The SCN receives light signals via a direct pathway from specialised photoreceptors in the eye (photosensitive retinal ganglion cells) which are most sensitive to blue light (Foster and Kreitzman, 2017). A reduction in brightness at dusk triggers the production of melatonin (the ‘vampire hormone’!) in the pineal gland, via the SCN. Although not a sleep-inducing hormone, melatonin serves as a cue for rest in humans, with levels increasing as we approach sleep and remaining high during the course of the night. In contrast, with dawn light, the SCN tells us it is time to be awake and melatonin levels are suppressed. The circadian clock responds differently to light depending on the timing of exposure, so that morning light advances the clock (making us get up earlier) and evening light delays the clock (making us get up later the next day) (Foster and Kreitzman, 2017). As well as playing a role in sleep regulation via the circadian system, light affects how alert you feel. You may have noticed that bright light increases your alertness, and so relatively bright light before bedtime will increase the likelihood that you will feel sleepier later that night than you might have done otherwise.
The second sleep system involves a homeostatic process. Put simply, the longer you have been awake, the greater the need for sleep will become. The homeostatic drive for sleep seems to be due to the build-up of various neurochemicals, for example adenosine, which increases while we are awake as a consequence of energy (adenosine triphosphate; ATP) use in the brain. Adenosine inhibits wake-promoting neurons in the basal forebrain and stimulates sleep-promoting neurons in the hypothalamus. As we sleep, adenosine is cleared and sleep pressure reduces.
The timing of sleep involves both of these systems as we would feel very sleepy mid-afternoon due to increasing homeostatic sleep pressure if it were not for an opposing circadian drive for wakefulness. Sleep occurs when the circadian drive for wakefulness ends and the homeostatic drive for sleep kicks in. Then, when the ‘sleep debt’ you have accumulated during the day has been re-paid, with an accompanying reduction in sleep pressure, and the circadian drive for wakefulness is sufficiently strong, we wake up.
Shedding light on teenage sleep
Biological, psychological and socio-cultural influences all play a part in the diminished sleep seen during adolescence (Carskadon, 2011). Teenagers are known to fall asleep and wake up at increasingly later times throughout this developmental period (Gradisar, Gardner and Dohnt, 2011). A tendency for a further delay to bedtimes at the weekend and a much later weekend wake time results in a widening disparity between the amount of sleep obtained on a school night compared with the weekend (Carskadon, 2011). Sleep requirements differ throughout the life cycle as well as from person to person. In 2015, the National Sleep Foundation recommended that teenagers, aged 14 to 17 years, should be getting eight to 10 hours’ sleep a night.
A review of studies across much of the globe concluded that teenagers are routinely not getting enough sleep, older adolescents obtain less sleep, and daytime sleepiness, a consequence of insufficient sleep, is prevalent in many countries (Gradisar, Gardner and Dohnt, 2011).
Sleep biology during the teenage years
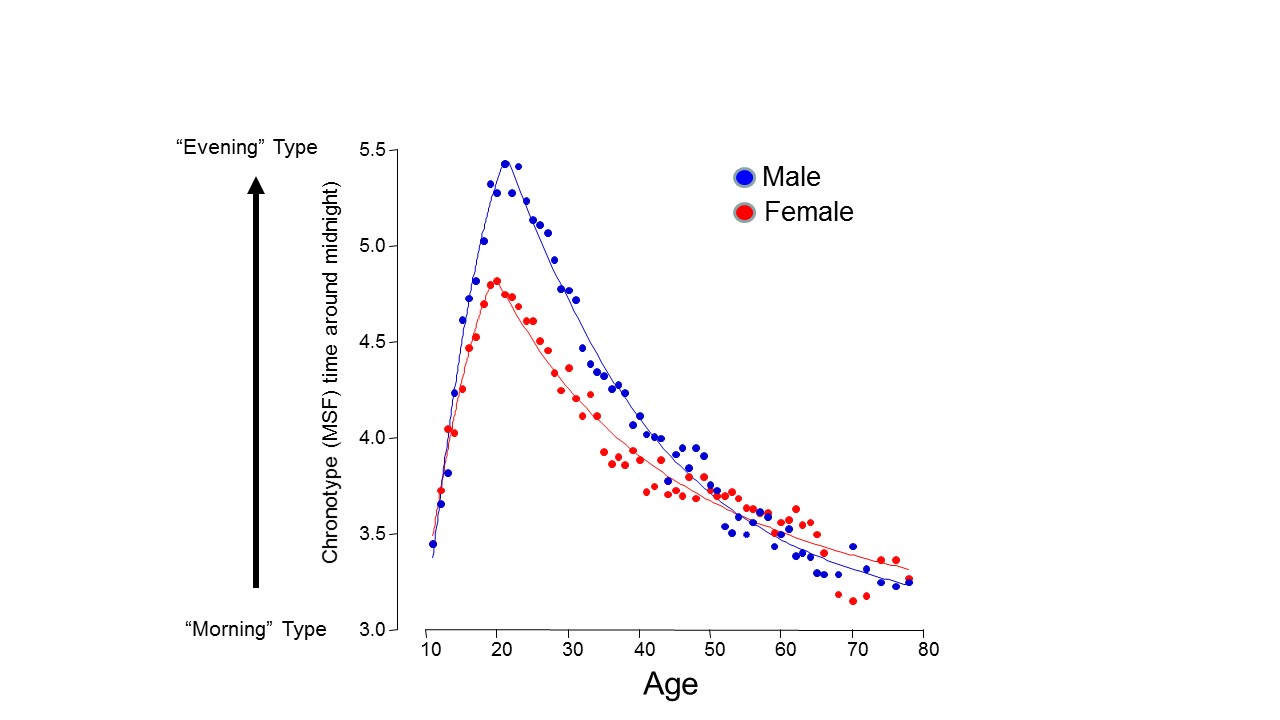
A developmental change in the two sleep processes occurs in adolescence. Sleep duration shortens drastically (by almost 2 hours) from the age of 10 to 17 (Fig. 1). Biological chronotype not only changes with age but also shows gender differences, delaying markedly through adolescence, as well as following the general pattern of females developing earlier than males, reaching its maximum at about 19.5 years in women and 21 years in men (Roenneberg et al., 2004). After this, chronotype advances so that the elderly have early sleep timing similar to children.
The circadian delay at puberty isn’t just confined to humans but has been found in a number of mammalian species, with a one to three hours’ delay in circadian phase in humans (Hagenauer, Perryman, Lee and Carskadon, 2009). This means that teenagers are biologically predisposed to going to bed late and getting up late. On a school night, teenagers may struggle to sleep at an early bedtime but they also need to wake up in time for lessons, which contributes to a reduction in total sleep time.
A couple of mechanisms have been suggested as the explanation for this shift in teenage circadian timing. The biological clock has been proposed to run more slowly at this age so adolescents have a longer circadian period. Alternatively, the SCN may have an altered sensitivity to light depending on whether it is dusk or dawn. Teens may experience a greater delaying effect of evening light yet a reduced advancing effect of morning light (Carskadon, 2011).
Sleep timing is also considered to be influenced by alterations to the sleep/wake homeostatic system. Sleep homeostatic pressure is thought to accumulate more slowly during adolescence than childhood, so the pressure to sleep builds up more slowly during the day (Carskadon, 2011). This helps to explain why a teenager is able to stay awake for longer than they could when they were a child.
Social jetlag
We know what jetlag feels like when we travel across time zones and how our internal time takes a while to adjust to the new external time. Teenagers experience something similar with ‘social jetlag’, when their internal time (chronotype) is misaligned with social time (school commitments), demonstrated by the disparity between sleeping in accordance with chronotype (weekend sleep) and sleep during the school week. Social jetlag isn’t confined to the teenage years but it is most pronounced in this age group. At the weekend, when a teenager is more likely to be able to go to bed and wake at their desired time (their chronotype), their bedtimes and wake times are slightly more than two hours’ later than during the school week (Gradisar, Gardner and Dohnt, 2011).
Psycho-social factors and sleep hygiene
Psycho-social factors interact with the bioregulation of sleep. Teenagers may be engaging in behaviours that are not conducive to routinely sleeping well. Improving sleep hygiene, the habits and practices that impact sleep, may help to minimise social jetlag and the delayed sleep intrinsic to adolescent sleep/wake timing. A couple of examples are described below which may have particular relevance for the teenage years.
The effect of light exposure on sleep has received increasing attention with the proliferation of electronic device use in recent years. However, the results are mixed. One study on young adults asked individuals to read an e-book at maximum intensity under dim room light for around 4 hours (18.00–22.00h) before bedtime on five consecutive evenings, whilst the control group read a printed book. The study concluded that those that read the e-book took longer to fall asleep, had lower morning alertness and a delay of the circadian clock compared with reading a printed book, but the effects were relatively small. Those participants who read e-books took only 10 minutes longer to fall asleep (Chang et al., 2015). As a result, some caution needs to be exercised when the press reports ‘reading an e-book in the hours before bedtime has unintended biological consequences that may adversely impact performance, health, and safety’ (Foster and Kreitzman, 2017).
Nevertheless, the impact of using electronic devices prior to sleep does seem to be important. In a large US survey, adolescents were shown to have increased the time spent using electronic media devices from 2009 to 2015. This was correlated with a decline in sleep duration (Twenge et al., 2017), and light from such devices has been blamed. Such studies have encouraged the use of in-built/downloadable applications that can dim the brightness of screens and/or reduce the light emitted. Light interventions are now being considered as a tool for adolescents experiencing difficulties with sleep, including reducing their exposure to evening light and increasing morning light to advance the clock to a time more suited for school. Unfortunately, the evidence showing that such interventions can be helpful is lacking. Furthermore, it is important to stress that game use, social media and watching TV are all likely to be cognitively arousing so teens may simply feel like staying up when they use electronic media devices. As a result, disentangling the light effects versus the alerting effects of devices is complex and awaits good experimental studies.
Caffeine is a stimulant with a half-life of approximately six hours in healthy adults. Caffeine blocks adenosine receptors in the brain, masking the build-up of homeostatic sleep pressure and consequently promoting wakefulness. Consuming caffeine later in the evening or excessive consumption may be another contributory factor to delayed sleep, and highly-caffeinated energy drinks are popular with teenagers. Correspondingly, the National Sleep Foundation 2006 ‘Sleep in America’ poll found that adolescents who drank two or more caffeinated beverages each day were more likely to have insufficient sleep on school nights and think they have a sleep problem, than those who drank one beverage or fewer.
Conclusions
Changes in the bioregulation of sleep predispose teenagers to experience insufficient sleep during the school week. This may be exacerbated by light exposure and sleep hygiene practices that impact sleep. Given the importance of getting enough sleep and its links to our health and wellbeing, sleep is an area which should be prioritised. Consequently, school-based interventions are now underway that aim to improve teenage sleep. Sleep education programmes inform teenagers about the lifestyle factors that may be affecting their sleep and how they can improve sleep hygiene. There is also a movement to delay school start times to better suit the adolescent clock by giving teenagers the opportunity to sleep at hours more aligned with their chronotype. So one could suggest that ‘early to bed, later to rise, may help the teen be healthy, wealthy and wise’ would be a better amendment to the age-old quote for our average teenager.
References
Borbély, A.A., 1982. A two process model of sleep regulation. Human neurobiology.
Carskadon, M.A., 2011. Sleep in adolescents: the perfect storm. Pediatric Clinics of North America, 58(3), pp.637-647.
Chang, A.M., Aeschbach, D., Duffy, J.F. and Czeisler, C.A., 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proceedings of the National Academy of Sciences, 112(4), pp.1232-1237.
Foster, R.G. and L. Kreitzman, Circadian Rhythms: A very short introduction. 2017: Oxford University Press, Oxford.
Gradisar, M., Gardner, G. and Dohnt, H., 2011. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep medicine, 12(2), pp.110-118.
Hagenauer, M.H., Perryman, J.I., Lee, T.M. and Carskadon, M.A., 2009. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental neuroscience, 31(4), pp.276-284.
Roenneberg, T., Kuehnle, T., Pramstaller, P.P., Ricken, J., Havel, M., Guth, A. and Merrow, M., 2004. A marker for the end of adolescence. Current Biology, 14(24), pp.R1038-R1039.
Twenge, J.M., Krizan, Z. and Hisler, G., 2017. Decreases in self-reported sleep duration among US adolescents 2009–2015 and association with new media screen time. Sleep medicine, 39, pp.47-53.
