
Physiology News Magazine
How nocturnal insects see in the dark
My journey studying the physiology of vision in nocturnal insects
Features
How nocturnal insects see in the dark
My journey studying the physiology of vision in nocturnal insects
Features
Eric Warrant
Lund Vision Group, University of Lund, Sweden
https://doi.org/10.36866/pn.102.31

My childhood experiences growing up in a rainforest led me to a career in Biology, and in 1985, as a new PhD student at the Australian National University in Canberra, I became surrounded by people who were studying insects, particularly those whose behaviours so deeply fascinated me as a child. What’s more, they were all doing research projects on the same topic – how insects see.
Ever since I was a child, growing up in an Australian rainforest, I have been fascinated by insects. Those in our garden – all manner of colourful butterflies, glistening beetles with endless jewel-like forms, and elusive slowly moving stick insects – captivated my imagination. Early on, it was their strange and wonderful colours and shapes that attracted me, but as I grew older, I began to see that they also had very interesting behaviours. Bees and flies could fly at breakneck speed without crashing, steering around trees to land. Large and venomous bull ants, the terror of every child’s life in the Australian bush, wandered great distances to and from their nests to scout for food. Mud wasps collected mud at the banks of our creek, and flew off out of sight to build their nest in a distant tree, only to return a short time later to collect more. It seemed to me amazing that insects could do such complex things. It still amazes me!
Not surprisingly, my experiences as a boy led me into a career studying insects, and particularly their vision and navigational abilities. Once in Canberra, I found myself in the Department of Neurobiology at the Research School of Biological Sciences, a world famous centre for insect vision led by a colourful character, Professor George Adrian Horridge, who had been recruited to his post almost two decades earlier from the University of St Andrews in Scotland.
Horridge is famous for his authoritative 1965 two-volume 1800 page classic Structure and Function in the Nervous System of Invertebrates, which he co-authored with Theodore Bullock. My project was to work on the physiological optics of superposition compound eyes, one of the two broad types of compound eyes found in insects, the other being the apposition compound eye. Unlike this latter type, superposition eyes – due to their especially sensitive optical design – are typical of nocturnal insects like moths and beetles. Apposition eyes, in contrast, are much less sensitive to light and are typical of day-active insects like bees, butterflies, dragonflies and flies.
Vision in the dung beetle
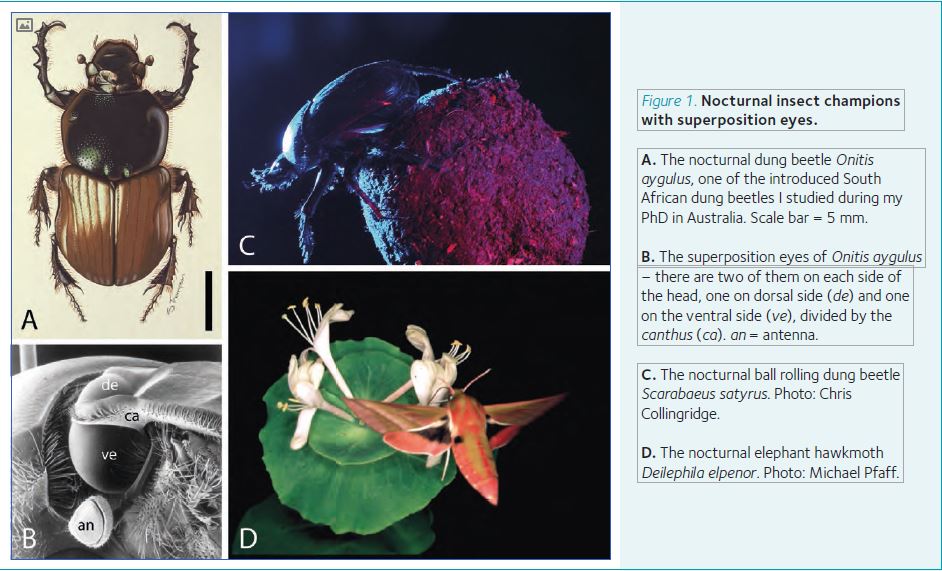
I was placed in the care of Dr Peter McIntyre, a mathematician who was developing optical ray-tracing models of superposition eyes using introduced South African dung beetles as a model system (Fig. 1A,B). These remarkable beetles were introduced into Australia about 20 years earlier by the Commonwealth Scientific and Industrial Research Organization (CSIRO) to rid Australia of the millions of wasted hectares of good pasture covered by cow manure every year.
Our local native species, evolved to deal with the dry fibrous pellets of Australian marsupials, were no match for the overwhelmingly large and moist discharges of our bovine imports. These dung beetles were a huge success, in fact one of Australia’s most successful biological control stories, quite unlike the notorious and catastrophic cane toad…
Under Peter’s expert guidance, I began to study dung beetle superposition eyes and to use electrophysiology to characterise the spatial and temporal acuity of their photoreceptors in an effort to find out how well this type of eye actually worked. Until then superposition eyes were thought to function as little more than sensitive low resolution imaging devices, sacrificing spatial resolution in order to maximise sensitivity at night. But the year before I started my PhD, Professor Mike Land from the University of Sussex (who was on sabbatical at Horridge’s department, but sadly left a few months before I arrived), used a home-built ophthalmoscope to measure the quality of images focused by the optics of superposition eyes in a day-active moth and a butterfly, two exceptional cases where superposition eyes have evolved for day use. He discovered that far from being blurry, these images were sharp and moreover diffraction limited (the best one could hope for in any optical system). My task was to find out whether this optical quality even extended to nocturnal superposition eyes, and whether the quality of the optical image was preserved in the neural image captured by the photoreceptor matrix.
Better-than-expected vision by superposition eyes
The answer it turned out was yes – nocturnal superposition eyes create reasonably sharp images (not quite diffraction limited though), and this image quality is preserved in the retina (Warrant and McIntyre 1990). In other words, it made me realise that nocturnal insects probably saw the world quite well! But how well? With such tiny eyes and brains, exactly how much information could they realistically extract from a dim nocturnal scene? Could what I had found in the retina really be used to create those sophisticated behaviours I had seen in day-active insects as a boy? What started out as a project in physiological optics had suddenly grown to a much broader interest in nocturnal vision and visual behaviour.
With this new interest I moved to Lund in Sweden, to take up a postdoc with Dr Dan-Eric Nilsson, the other world-leading expert on the optics of compound eyes (the other being Mike Land – he and Dan have since written a beautiful and warmly recommended book describing the great variety of eyes found in nature, simply titled Animal Eyes (Oxford, 2012)).
While building up my new equipment (a brand new electrophysiology lab with a sophisticated optical stimulation system), I became increasingly curious about how well animals actually might see in very dim light.
Vision in very dim light
It occurred to me that nocturnal animals might be able to sum photons in space and time to increase sensitivity, akin to pooling pixels to create a larger and more sensitive pixel, or on a camera lengthening the exposure time. This would require neurons at some higher level in the visual system that sum the photoreceptor signals coming from small groups of neighbouring ommatidia (the optical building blocks of compound eyes, each consisting of a lens-pair and an underlying bundle of photoreceptors). Since each ommatidium is responsible for sampling a single pixel of the visual scene, this neural spatial summation would create a large ‘super ommatidium’ that samples a large ‘super pixel’. Similarly, some higher neuron, or circuit of neurons, might be responsible for lengthening the visual exposure time (which is equivalent to the visual integration time).
The downside of such a summation strategy is, however, that fewer larger pixels reduce spatial resolution, and a longer exposure time reduces temporal resolution. In other words, to improve sensitivity one would need to throw away the finer and faster details in a visual scene in order to see the coarser and slower ones a lot better. But this might be better than seeing nothing at all! With this realisation, I started to build a mathematical model to calculate the finest spatial detail that a nocturnal animal, with a given eye design, might see using such a summation strategy as light levels fell (Warrant, 1999).
The results were surprising – spatial and temporal summation should in theory allow nocturnal animals to see at light levels several orders of magnitude dimmer than would have been possible had summation not been used. But could nocturnal animals actually do this? The benefits of summation seemed obvious, and I became convinced that it must be a crucial component of nocturnal visual processing.
Summation allows vision in very dim light with video too
I also realised that the same strategies could be used to improve video filmed in very dim light, and quite out of the blue, not long after I had published my model, I was contacted by the car manufacturer Toyota (who had realised the same thing). Toyota were very keen to develop an in-car camera system that could automatically monitor the road ahead at night – using only the existing natural light – and warn the driver of impending obstacles.
That led to a long collaboration in which we developed mathematical computer algorithms that could be used to dramatically improve the quality of video sequences captured in dim light (Warrant et al., 2014), and as far as we know Toyota are now using this technology to develop camera systems for their cars. However, from our point of view the results of this exercise were telling – summation could improve the quality of dim video sequences dramatically, even restoring colour information! This convinced me further that summation could make a real difference for vision in dim light.
That chance call from Toyota was not the only out-of-the-blue event from that time that dramatically drove our research on nocturnal vision forward. A couple of years earlier I was invited by Professor Clarke Scholtz, South Africa’s preeminent expert on beetles, to talk about the visual system of dung beetles at the first (and only) International Conference on Scarab Biology to be held in the beautiful Kruger National Park north of Johannesburg. One of the lectures – by Dr Marcus Byrne from the University of the Witwatersrand – dealt with the behaviour of ball-rolling dung beetles. These beetles fashion golf-ball sized balls of dung and then roll them away backwards for burial (prior to which a female deposits her egg at the ball’s centre – the dung is used as food by the developing beetle). At one point in the lecture Marcus told of how beetles rolled their large balls in almost perfectly straight lines (presumably to most efficiently escape the competitive fury of the dung heap), but was completely unable to explain how they managed this seemingly impossible task.
How do insects navigate a straight line?
Sitting in the audience, I thought I might know the answer – many other insects are able to see the circularly symmetric pattern of celestial polarised light formed around the sun and to use it as a compass cue for orientation and navigation. Maybe dung beetles could too? Half a century earlier, Karl von Frisch, the great Austrian zoologist who had discovered the dance language of honeybees, also discovered that insects used celestial polarised light for orientation. Since that time, we now know that a wide variety of animals, particularly invertebrates, can see and use this celestial cue (mammals like ourselves, however, are not among them). A good chat with Marcus after his lecture was the start of a long and very fruitful collaboration that has continued to this day. As it transpired, not only do the day-active species roll in straight lines (and indeed rely on the solar polarisation pattern), but so too do the nocturnal species. These, it turned out, instead rely on the million times dimmer polarisation pattern formed around the moon as a compass cue. This remarkable navigational ability indicates that these dung beetles have exquisitely sensitive nocturnal vision.
After many trips to the wilds of South Africa with Marcus and Clarke – now in the company of my good friend Dr Marie Dacke from Lund, who these days runs this project – we discovered that not only do nocturnal beetles like Scarabaeus satyrus (Fig. 1C) have sufficiently sensitive superposition eyes to see and use the dim lunar celestial polarisation pattern to roll in straight lines (Dacke et al., 2003), but even use the faint stripe of starry light formed by the Milky Way as a back-up when the moon and its polarisation pattern are absent from the sky (Dacke et al., 2013)! To prove all this we resorted to methods, including designing and producing small cardboard hats we could tape to the head of the beetle and which blocked their view of the sky (but not of the terrestrial surround). Hatted beetles just rolled their balls around in circles. To show their dependence on the Milky Way on moonless nights, we moved the beetles into the Johannesburg Planetarium where we could turn the stars and the Milky Way on and off at will. We were surely the strangest visitors the planetarium had opened its doors to, but we were enthusiastically accommodated nonetheless. The IgNobel prize committee was also enamoured by our efforts and in 2014 they awarded us their first (and probably their last) IgNobel prize jointly in the fields of Astronomy and Biology! Since then we have discovered exquisitely sensitive neurons in the dung beetle central brain that are responsible for coding nocturnal compass information and for establishing the hierarchy of cues used in straight-line navigation (Jundi et al., 2015).

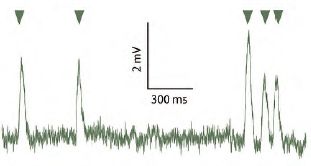
Figure 2. The nocturnal sweat bee Megalopta genalis from Central America (above). Recordings of photoreceptor responses to single photons of light (arrow heads) in Megalopta (green trace), as well as in the closely related day-active species Lasioglossum leucozonium (red trace). Note that the amplitudes of the receptor responses in the nocturnal species are about 5 times the amplitude of those in the day-active species, an adaptation in nocturnal insects that increases visual reliability in dim light.
Nocturnal bees in Panama
Not too long after the conference in Kruger National Park I received a phone call – again out of the blue – from Dr Bill Wcislo, a well-known bee ecologist from the Smithsonian Tropical Research Institute in Panama. He just wanted to inform me, in case I didn’t know, that in Panama there were strictly nocturnal bees that foraged in the rainforest at night, and that maybe I would be interested in studying their vision. I was flabbergasted. I had never heard of strictly nocturnal bees, and of course I was interested! And we have been studying these amazing bees – sweat bees of the genus Megalopta (Fig. 2) – ever since. Together with my first postdoc Dr Almut Kelber (now a full professor in Lund), we discovered that these bees have remarkable nocturnal visual powers. Like their day-active relatives, Megalopta uses its eyes to learn visual landmarks around its nest (a small hollowed out stick in the rainforest undergrowth), as well as along the foraging route, and uses these landmarks to find its way home after a successful foraging trip (Warrant et al., 2004). It does this at perishingly low light levels – we ourselves are almost totally blind, yet these bees are negotiating branches, tree trucks and bushes while flying through the forest understorey. What makes this all the more incredible is that they are doing it with apposition compound eyes – the compound eye design typical of insects active in bright light (including all other bees). By recording the responses of their photoreceptors to single photons (Fig. 2), we determined that at the light intensities Megalopta are active, each photoreceptor on average absorbs fewer than 10 photons per second. This is absurdly little light, in fact according to theory it should be at least 100 times too little for the bee to see its nest entrance! Yet it does, and we have since discovered that at these light levels it can fly and land on its nest with the same speed and precision as a day-active honeybee. How? Again visual summation likely provides the answer. We are currently investigating this possibility by studying their visual processing using two recently built state-of-the-art electrophysiology labs in Panama.
The nocturnal elephant hawkmoth can see colours at night
After years of trying to find proof for the existence of summation in the neural circuitry of the visual system, we have finally found it in the optic lobes of the nocturnal elephant hawkmoth Deilephila elpenor (Fig. 1D). These beautiful moths have very sensitive superposition eyes, and are accomplished fliers; being able to suck nectar from flowers on the wing, while hovering to keep station. Some years ago, Almut and I had discovered that these moths have trichromatic colour vision, and use it to locate flowers at night, the first nocturnal animal known to have colour vision (Kelber et al., 2002). In more recent years, a talented PhD student in my group, Anna Stöckl, has managed to record from wide-field motion-sensitive cells in the lobula plate region of the moth’s optic lobe, the brain region responsible for processing visual information. These visual cells – well known in other insects, particularly flies – are responsible for analysing the way the visual image of the world moves as an animal moves through it. An accurate analysis of this so-called ‘optic flow’ is essential for course control and obstacle avoidance, especially in a fast flying animal like a moth.
The responses of these neurons to moving patterns of black-and-white stripes (known as ‘gratings’), at increasingly dimmer light levels, can be used to test for the presence of summation. By making the stripe width thinner and thinner, and by moving the grating faster and faster, one can measure the finest spatial and temporal details that the motion cells can resolve at each light level and then use the same stimulation regime to compare these to the finest details resolved by the retina (the visual input). Any coarsening or slowing of vision at the level of the motion-sensitive lobula plate cells relative to that in the retina would then indicate the presence of summation somewhere in the intermediate visual pathway. Using this method, Anna has found that substantial spatial and temporal summation occurs prior to the lobula plate. This summation increases as light levels fall, dramatically boosting visual reliability for courser and slower details in the scene over four decades of nocturnal light intensity. Moreover, without summation the moths would become blind at light levels at least 100 times brighter than they actually do. These findings provide the long-sought proof of the benefits of summation for vision in dim light.
But even though we have now probably pinned down summation as the major player in extending the limits of vision in dim light, it doesn’t detract from the fact that insects, and in particular nocturnal insects, are truly amazing. My boyhood fascination for them has not in any way diminished with the years. To the contrary, my fascination has only increased. To know that a small nocturnal bee, with tiny apposition eyes and a rice-grain-sized brain, can find its way to a lucrative source of flowers through the complicated tangle of a rainforest at night, and then find its way home again, is both humbling and awe-inspiring. For us to witness this spectacle requires a pair of night vision goggles and infrared illumination, and even during the day we would become hopelessly lost in this jungle if we strayed even a few metres from the trail. How a bee manages to forage and home at night is still largely a mystery, and despite all our work we are still only a fraction of the way to understanding the physiological solutions that these and other remarkable nocturnal insects use in order to see, fly and navigate at night.
References
Dacke M, Nilsson, DE, Scholtz CH, Byrne M & Warrant EJ (2003). Insect orientation to polarized moonlight. Nature 424, 33
Dacke M, Baird E, Byrne M, Scholtz C, and Warrant EJ (2013). Dung beetles use the Milky Way for orientation.
Current Biology 23, 298-300
Jundi B el, Warrant EJ, Byrne MJ, Khaldy L, Baird E, Smolka J and Dacke M (2015). Neural coding
underlying the cue preference for celestial orientation.
Proc Nati Acad Sci USA 112 (36), 11395-11400
Kelber A, Balkenius A & Warrant EJ (2002).
Scotopic colour vision in nocturnal hawkmoths.
Nature 419, 922-925
Warrant EJ (1999). Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation.
Vision Research 39, 1611-1630
Warrant EJ and McIntyre PD (1990). Limitations
to resolution in superposition eyes.
J Comp Physiol A 167, 785-803
Warrant EJ, Kelber A, Gislén A, Greiner B, Ribi W
& Wcislo WT (2004). Nocturnal vision and landmark orientation in a tropical halictid bee.
Current Biology 14, 1309-1318.
Warrant EJ, Oskarsson M and Malm H (2014).
The remarkable visual abilities of nocturnal insects: Neural principles and bio-inspired night-vision algorithms.
Proc IEEE 102, 1411-1426.
