
Physiology News Magazine
Aquatic and aerial animal athletes
Adaptations for muscle power and speed in tuna and hummingbirds
Features
Aquatic and aerial animal athletes
Adaptations for muscle power and speed in tuna and hummingbirds
Features
Timothy G West
Structure & Motion Laboratory, Royal Veterinary College, UK
https://doi.org/10.36866/pn.102.36

Differences in skeletal muscle structure and function contribute to the diversity in animal locomotor capacity and at least partly explain remarkable feats of animal speed (like the flat-out hunting swims in tuna) and stamina (like fish and bird migrations). Integrative experimental approaches needed to study muscle physiology in specialised animal athletes are helping to refine our understanding of these mechanisms, including in relation to muscle function in humans. Ongoing analyses will also sharpen our predictions of how environmental changes might impact on migration patterns, hunting success, (re)distribution and survival of peak performers in the wild.
Decades of comparative animal biology have revealed remarkable diversity in animal activity metabolism. There are examples of swimmers, flyers and runners that are legitimate elite athletes, although it is clear that, compared with human athletics, it is chiefly the processes of evolution and survivorship that shape athleticism in wild animals, rather than routine training and motivational accolades. Comparative researchers are drawn to measurements of animal speed, power, stamina and metabolism, partly with the aim of advancing our understanding of the physiological limits and environmental constraints for wild animals living in diverse, and changing, habitats. A key aim is also to seek the basic features of striated muscle design that underlie speed and endurance adaptations across species, including humans.
Animal performance is popular to review. Recent excellent reviews by Sharp (Sharp 2012) and Williams et al (Williams et al., 2015) comprehensively summarise the diversity of particularly mammalian and avian athleticism, including useful speed and endurance statistics and comparisons with human performance limits. Our understanding of behaviours which might normally be overlooked as athleticism, such as mechanisms of dive-time endurance and extreme cardiac function in marine mammals, are useful for highlighting the physiological limits of organ-level function and possibly for predicting the circumstances when physiological demands might exceed performance capacity in humans (Williams et al., 2015). Similarly, recent work shows us that skeletal muscle adaptations for speed and endurance in elite swimmers (tunas) and flyers (hummingbirds) can inform us about the limits and extremes of muscle plasticity and locomotory performance.
Tuna Swimming Muscles: adapted for power
Arguably, the swimming musculature of fast swimming fishes, including tunas, billfishes and lamnid sharks (e.g. great whites and makos) is the ultimate in top performance design. The tunas, for example, display both fast sustainable cruising swims and super-fast anaerobic (hunting) bursts. While the capacity for performing both aerobic and anaerobic work is not unique amongst animal athletes, it is rare to find a generalised top-performance phenotype. The two extremes of human anaerobic and aerobic training tend to develop either the muscly high-power sprinter or the lean long-distance endurance racer. In contrast, tuna swimming musculature is adapted for high aerobic, and higher still anaerobic, power output. The flexible performance capacity of tunas, together with the anatomical separation of fibre types into distinct red (aerobic cruising fibres) and white (glycolytic, burst fibres) muscle masses, makes tuna an ideal model for investigating the fibre-type dependent features of peak power and speed.
Many factors contribute to sustainable aerobic performance. Tunas are streamlined Thunniform swimmers, using high frequency oscillations of the hydrofoil shaped tail, rather than rhythmic body undulations of the trunk musculature, for high speed cruising. The red muscle is an aerobic power-house; (i) there is a lot of it compared to other fishes, and oxygen delivery, fibre capillarity and mitochondrial function are all relatively high for fishes, (ii) the anterior and medial position of red muscle within the trunk, rather than in the peripheral mid-line area, confers a biomechanical advantage for high power caudal oscillations, and (iii) red muscle temperature is typically 10°C above ambient sea temperature, so pathways of energy demand and supply operate at the upper end of their temperature dependencies (Mathieu-Costello et al., 1995; Moyes and West 1995; Shadwick and Syme 2008). It seems that optimal red muscle contractions, tested in vitro, are likely to support cruising at or near peak aerobic power (Shadwick and Syme 2008). This may constrain tunas to a relatively narrow, high-performance-only, lifestyle (discussed later). Anatomical measures of tuna red muscle aerobic capacity (eg., fibre cross-sectional area, fibre capillary length, volume density of mitochondria) are not all outstanding compared to those of athletic and non-athletic mammals and birds (Mathieu-Costello et al., 1995), suggesting that red muscle endothermy and the biomechanical advantage of its medial-positioning are the main aspects of tuna aerobic cruising performance.
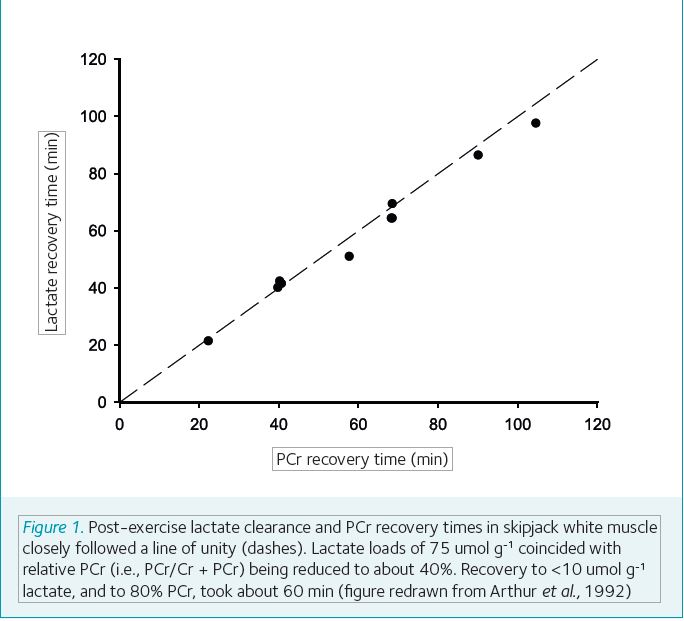
Fewer factors contribute to the maximal burst power in tuna. Maximal bursts of 20 body-lengths s-1 are supported by anaerobic contractions of a large white muscle mass and are dependent on the breakdown of intramuscular glycogen, phospho-creatine (PCr), and adenosine triphosphate (ATP). Glycogen content of skipjack tuna white muscle is about 150 µmol g-1 (Arthur et al., 1992), and glycolytic enzyme activities are some of the highest measured in vertebrates (Moyes and West 1995). Different durations of maximal swimming result in variable levels of lactate accumulation, but the molar stoichiometry of glycogen-used:lactate-formed is nearly 1:2. Remarkably, lactate accumulation of >100 µmol g-1 occurs in skipjack white muscle (Arthur et al., 1992), and this is still only one-third depletion of the total glycogen store. The stoichiometry between glycogen breakdown and lactate build-up highlights that maximal power is not dependent on either circulatory oxygen or glucose. Human sprinters and power athletes are similarly poised for anaerobic power output, but with lower glycolytic capacity than tuna. Interestingly, the intramuscular handling of metabolites in tuna persists during exercise recovery.
Figure 1 depicts recovery times for lactate and PCr in post-exercise tuna white muscle. Intramuscular pH regulation, together with high creatine kinase activity, is the likely mechanistic link between muscle lactate removal and PCr replenishment; proton removal accompanies lactate disappearance in recovery and, in turn, pulls the creatine-kinase equilibrium in the direction of phosphate exchange from ATP to Cr. Muscle lactate clearance via the circulation and by oxidation, as occurs after maximal exercise in mammals, seems insignificant in tuna (Arthur et al 1992, Moyes and West 1995). Nevertheless, lactate, PCr and glycogen all recovery rapidly in tuna, despite relatively low temperatures, and even after accumulation of large lactate loads. Lactate disappearance and glycogen restoration in recovery show an approximately 2:1 stoichiometry, mirroring the metabolic state seen during anaerobic contractions (Arthur et al 1992), and suggesting activity of chiefly an intramuscular pathway of lactate-to-glycogen conversion during recovery.
Tuna white muscle is an extreme phenotype, but there are useful benchmarks for improving human performance; including, the large muscle mass and high glycolytic capacity needed for burst power, the marked intramuscular management of fuels and metabolites, and rapid post-exercise recovery. Different factors control the fate of lactate and speed of recovery in tunas and trained humans, but both recover fast – humans so they can train often to get better and tunas so they can hunt often.
Hummingbird flight muscle: adapted for aerobic fuel efficiency
Hovering/feeding flight in hummingbirds may be the near-limit of striated muscle shortening speed and fuel oxidation rate. Aerobic metabolic rates during hovering are some of the highest values measured among the vertebrates, and it is estimated that the fast-twitch oxidative flight muscle accounts entirely for the metabolic costs of hovering. The evolution of heightened functionality at multiple steps in the lung-to-mitochondria oxygen cascade (e.g., lung diffusion, cardiac output, muscle mitochondrial volumes) seems to underpin the efficiency of oxygen flux, and fuel (fat and carbohydrate) usage, during hovering flight (Suarez et al., 2011). carbohydrate quickly becomes the preferred fuel for muscle activity during subsequent feedings. The transition between fuel types is interesting because it occurs without a change in work intensity – hovering flight is hovering flight. A fat-to-carbohydrate transition in exercising mammals usually accompanies a change from low to high aerobic metabolic rate. The hummingbird result shows that fat is actually suitable for peak aerobic muscle work, but if both fuels are available for flight then carbohydrate is preferred. Flexibility in fuel selection is important for hummingbirds, as they will undoubtedly use both fat and carbohydrate during migration. However, in the non-migratory state, the shift towards carbohydrate promotes (i) efficient exploitation of carbohydrate-rich nectar and (ii) efficient oxygen usage – the yield of ATP per atom of oxygen is higher when muscle is burning glucose than it is when burning fatty-acids (Suarez et al., 2011).
The second remarkable aspect of hovering flight is that fuel (nectar) intake supports muscle energy supply (i.e., glucose oxidation) very soon after ingestion. Up to 95% of the metabolism of hovering flight is fuelled by recently ingested sugar. Suarez et al., (2011) call the flow of sugar, from flower to muscle mitochondria, the ‘sugar oxidation cascade’. Good synchrony between glucose and oxygen delivery to muscle is important for matching energy demand (contractile ATP use) with energy supply (mitochondrial ATP synthesis) and for maintaining oxidative efficiency with a carbohydrate-based metabolism. The nectar bats seem to have also evolved the strategy for glucose uptake and oxidation (Suarez et al., 2011). Similar supply-demand balance must also characterise steady-state cruising in tunas and endurance exercise in humans, but with greater dependence on ‘on-board’ fuel stores and perhaps less clear-cut patterns of carbohydrate oxidation.
It seems clear that exogenous glucose, via the sugar oxidation cascade, is the main ATP source for hovering flight. An advantage of the sugar oxidation cascade in hummingbirds is that any energetic burden of carrying on-board fuel masses is minimised. The day-to-day risk of this strategy, and even longer-term danger due to habitat change, is that the animal needs to locate fuelling-up sources quickly. The capacity to fuel costly hovering flight with on-board fat is one safety factor that the birds can exploit in the short term.
Integrating muscle energy supply and demand with animal locomotion
Near the end of PW Hochachka’s book Muscles as Molecular and Metabolic Machines, he writes that ‘The full power of the reductionist approach is realized only when it is reincorporated into the integrationist one’ (Hochachka 1994). A good example is how we integrate the mechanisms of muscle energy supply with muscle energy demand and, in turn, integration of muscle energetics with animal locomotion. Muscle energy balance (supply matching demand) is a key to maintaining flexible muscle performance for both migration and foraging/hunting success in the wild. Comparative or ecological physiologists would naturally extend the meaning of Hochachka’s point to include the role for plasticity in activity capacity in the face of environmental change.
Tuna and hummingbirds possess remarkable features of muscle energy supply, including oxygen and fuel delivery rates, fuel oxidation capacities, and high glycolytic enzyme activities. Integration with energy demands (cross-bridge and activation/deactivation kinetics and costs) is critical, since it is these properties that dictate muscle force, speed and power, and in turn drive the upregulation of energy supply. Hummingbirds and tunas are ideal models for studying the integration of energy supply and demand because (i) fibre-types are either entirely homogenous (hummingbird fast-twitch oxidative flight muscle) or segregated into functionally distinct fibre masses (tuna red and white swimming muscles), and (ii) both tuna cruising and hummingbird hovering are near-peak aerobic activities, so it is relatively straightforward to integrate muscle power with specific patterns of real-world locomotion and with metabolic rates.
Cruising in tuna, during schooling and migration, is not only normal behaviour it is also essential life-support. Tuna match high aerobic demand with efficient oxygen supply from water; they do so by swimming continuously, with mouth wide open, in order to ‘ram ventilate’ the gills. While different tuna species have different preferred cruising speeds, it seems that optimal red muscle contractile demands are likely to support cruising at a very high level of aerobic power (Shadwick and Syme 2008). This level of muscle demand/supply integration is possible because in vivo tail beat frequency and amplitude can be compared with, or directly replayed onto, movement patterns in isolated muscle preparations. The finding that red muscle activity may keep tunas at or near peak power output in vivo is interesting because rhythmic activation of the red muscle itself must be a major component of the metabolic demand associated with ram ventilation.
Cruising at a high level of aerobic power seems in a sense to be a costly mode of locomotion. This is possibly offset partially by muscle operating in vivo in a zone of peak efficiency. Moreover, a major trade-off is that high speed coupled with a degree of red muscle thermoregulation expands the tunas’ range of locomotion and hunting habitats. The impact of ocean temperature-change on the capacity for habitat expansion needs to be explored. Interestingly, similar red muscle anatomical design has evolved independently in fast swimming tunas and lamnid sharks, indicating that a high-power design, together with mechanisms to keep cruising muscle warm, is a superior phenotype amongst the top pelagic predators.
There are not many studies of hummingbird isolated muscle mechanics. A recent study found that demembranated (or ‘skinned’) single fibres generated low isometric forces per cross-sectional area (CSA), perhaps indicating that low stress favours high shortening speed in this special system (Riser et al., 2013). This fits with the suspected rapid calcium kinetics which should keep twitch forces low and muscle cycling rate high, and with observations of high mitochondrial volume density relative to myofibril volume in hummingbird flight muscle (Mathieu-Costello et al., 1995; Riser et al., 2013).
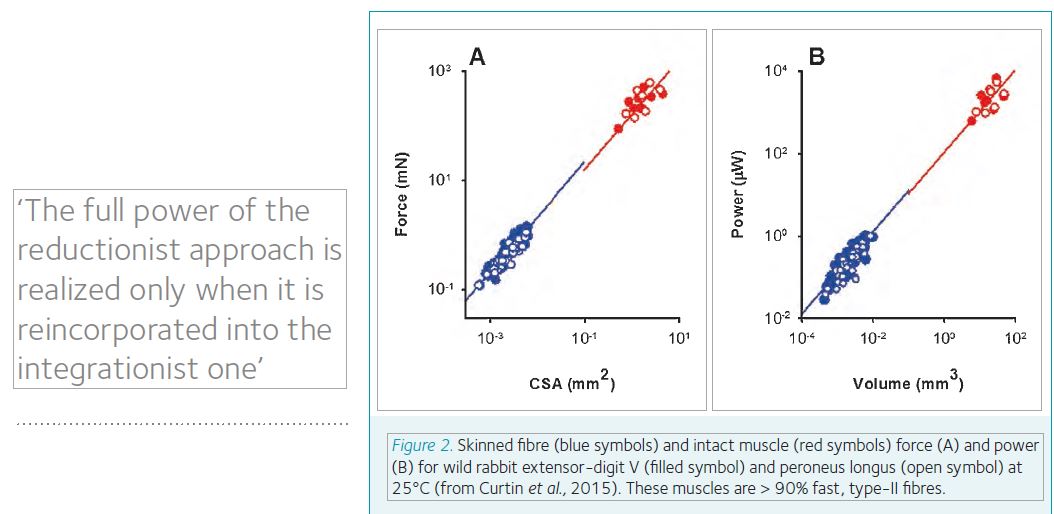
It would be of further interest to measure directly the muscle shortening properties in order to characterise fibre force and velocity at peak power. Physiologically relevant length changes are expected to be quite short in hummingbird flight muscle because of the rapid cycling time and short fibre lengths. This could make measurements with skinned hummingbird fibres challenging, especially at high temperatures. One way forward is to make measurements of length changes during force-clamp after temperature-jump activation of the skinned fibre. The temperature-jump approach keeps skinned fibre activation time short, and allows for more repeatable activations at elevated temperatures. Recent work integrating skinned and intact (electrically excitable) muscle mechanics, in the same lab and using the same muscles from wild rabbit, shows that isometric forces and peak powers at 25°C are equal in the two kinds of preparation (Fig 2; Curtin et al., 2015). Oxygen consumption during hovering flight seems to interrogate a peak energy supply/demand steady-state of primarily the flight muscle of hummingbirds (Suarez et al., 2011). Since the demands are expected to be largely ATP usage for cross-bridge turnover and calcium handing, it would be valuable to integrate direct measurements of skinned and intact muscle power with the in vivo hovering flight physiology.
Together with the emerging ideas about a sugar oxidation cascade, this work could make the elite hummingbird one of the most completely integrated models of vertebrate muscle mechanics and energetics.
References
Arthur PG, West TG, Brill RW, Schulte PM & Hochachka PW (1992). Recovery metabolism of skipjack tuna (Katswonus pelamis) white muscle: rapid and parallel changes in lactate and phosphocreatine after exercise. Can J Zool 70, 1230-1239
Curtin NA, Diack RA, West TG, Wilson AN & Woledge RC (2015). Skinned fibres produce the same power and force as intact bundles from muscle of wild rabbits. J Exp Biol 218, 2856-2863
Hochachka PW (1994). Muscles and Molecular and Metabolic Machines. Boca Raton, Florida: CRC Press
Mathieu-Costello O, Brill RW & Hochachka PW (1995). Design for a high speed path for oxygen: tuna red muscle ultrastructure and vascularization. In: Hochachka
PW & Mommsen TP (eds.) Biochemistry and Molecular
Biology of Fishes, Vol 4 Metabolic Biochemistry.
(pp. 1-13). Amsterdam, The Netherlands: Elsevier
Moyes CD & West TG (1995). Exercise metabolism of fish. In: Hochachka PW & Mommsen TP (eds.) Biochemistry and Molecular Biology of Fishes, Vol 4 Metabolic Biochemistry. (pp.367-392).
Amsterdam, The Netherlands: Elsevier
Riser PJ, Welch KC, Suarez, RK & Altshuler DL (2013). Very low force-generating ability and unusually high temperature dependency in hummingbird flight muscle fibres. J Exp Biol 216, 2247-2256
Shadwick RE & Syme DA (2008). Thunniform swimming:
muscle dynamics and mechanical power production of aerobic fibres in yellowfin tuna (Thunnus albacares).
J Exp Biol 211, 1603-1611
Sharp CNC (2012). Animal athletes: a performance review. Veterinary Record 171, 87-94
Suarez RK, Herrera M LG & Welch Jr KC (2011).
The sugar oxidation cascade: aerial refuelling in hummingbirds and nectar bats. J Exp Biol 214, 172-178
Williams TM, Bengston P, Steller DL, Croll DA & Davis RW (2015). The healthy heart: Lessons from nature’s elite athletes. Physiology 30, 349-357
