
Physiology News Magazine
You are what your mother ate
The effects of maternal obesity during pregnancy on offspring obesity and cardiovascular diseases
Features
You are what your mother ate
The effects of maternal obesity during pregnancy on offspring obesity and cardiovascular diseases
Features
Laura Dearden & Susan E Ozanne
University of Cambridge Metabolic Research Laboratories & MRC Metabolic Diseases Unit, Wellcome Trust-MRC Institute of Metabolic Science, Cambridge, UK
https://doi.org/10.36866/pn.106.26
The obesity epidemic in the developed world is hard to escape. Media coverage and observations from everyday life emphasise the rise in obesity in the UK across all ages and socio-economic classes. By 2050, obesity is expected to affect 60% of adult men and 50% of women. The rapid increase in obesity is shortening average lifespan and has enormous financial implications for society. Much of the latter comes from treatment of type 2 diabetes, which is largely associated with an increased body mass index (BMI).


Obesity-generated type 2 diabetes now accounts for nearly 90% of the total diabetes diagnoses in the UK. It was revealed recently that the cost of treating diabetes in England accounts for 10% of annual NHS prescribing costs, or about £2.2 million on average every day in 2013-14. One in seven hospital beds are occupied by someone with diabetes. The rapid rise in obesity has occurred – in evolutionary terms – over a very short period of time. Whilst several genetic polymorphisms associated with obesity have been identified, these only account for small increases in body weight and explain a small amount of the heritability of the condition. Therefore the changing environment is thought to be a major driver of the obesity epidemic. We are living increasingly sedentary lifestyles. Recent surveys show that over one in four females and one in five males in the UK are classified as ‘inactive’ because they undertake less than thirty minutes of physical exercise a week. This combined with the widespread availability of highly palatable calorie-dense fast food is inevitably fueling a rise in body weight. In addition to these genetic and lifestyle factors, it has recently become clear that the early life environment is also important in shaping the risk of obesity in later life.
Environment and metabolic disease
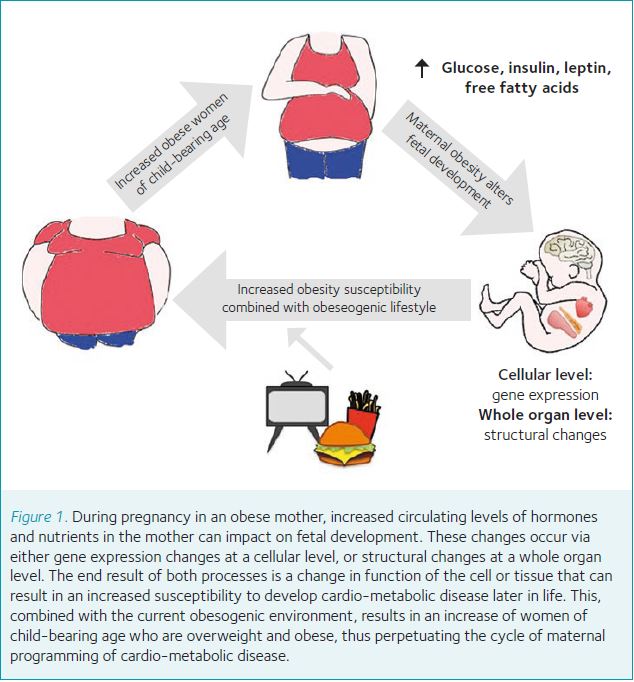
An interaction between the early life environment and later life metabolic disease risk was first proposed in the seminal papers by Hales and Barker in the early 90s, who reported an association with low birth weight (used as an indicator of reduced fetal growth) and cardio-metabolic disease in adulthood in a Hertfordshire UK Birth Cohort. Further studies examining individuals who were in utero during the Dutch Hunger Winter, a six-month famine in the Netherlands during the Second World War, confirmed the association between in utero under-nutrition and the development of cardio-metabolic disease. Due to the rapid onset and abrupt end of the famine, studies of individuals who were in utero during the famine have provided an invaluable insight into the effects of exposure to under-nutrition exclusively during the in utero period. Individuals who were in utero during the famine display a range of disease phenotypes as adults, with many of the phenotypes varying depending on sex and the time of exposure to the famine (i.e. early or late gestation). As well as the detrimental effects of exposure to under-nutrition in utero, there is now a wealth of evidence that demonstrates early life exposure to over-nutrition – for instance in cases of maternal obesity or diabetes during pregnancy – is also associated with increased risk of the same cardio-metabolic diseases. This is particularly concerning as it is estimated that about half of women of childbearing age in England are either overweight or obese. The prevalence of obesity in women of this age bracket increased from around 12% in 1993 to over 19% in 2013 and is predicted to rise further.
The consequences of maternal obesity on health of the offspring
It is hard to distinguish between obesity due to inheritance and obesity resulting from the uterine environment. Therefore, studies comparing siblings with different maternal exposures (be it maternal obesity or diabetes) are imperative. Studies of siblings born before and after the mother underwent gastric bypass surgery have revealed that the children born after the mother had lost weight have reduced adiposity and improvements in insulin sensitivity compared to their siblings born prior to surgery (Guenard et al., 2013). Similarly, with siblings of diabetic mothers, the sibling who was in utero when the mother was diabetic has an increased risk of developing type 2 diabetes later in life. Of course as well as sharing a similar genetic make-up, children within the same household will often have similar current lifestyles (i.e. diet and exercise habits) and therefore any differences in their body weight and diabetes risk are likely to be a result of their different in utero environments. To fully control these extra variables that can impact on obesity risk, researchers have used animal models of maternal obesity in which the genetic background and diet of the mother and offspring can be tightly controlled.
Animal models
Increased weight gain in offspring exposed to maternal obesity in utero is often preceded by increased food intake. This suggests altered neural regulation of energy homeostasis as an underlying cause of metabolic phenotypes. Central control of food intake can be broadly divided into two areas: homeostatic control originating in the hypothalamus, and reward-related feeding behaviour orchestrated through the mesolimbic pathways. The main regulators of feeding behaviour in the hypothalamus are orexigenic (appetite stimulating) neurons expressing Neuropeptide-Y (NPY) and Agouti-Related Peptide (AgRP) and anorexigenic neurons expressing Pro-opio Melanocortin (POMC). Rodent studies have shown that exposure to maternal obesity and diabetes in utero causes changes not only to the numbers of these neurons but also to their axonal projections. Changes in these vital neuronal circuits are associated with hyperphagia in the offspring. The feeding circuits in the hypothalamus may be particularly vulnerable to disruption by maternal obesity as their embryonic development is orchestrated by the precise levels of metabolic hormones, in particular leptin and ghrelin (Dearden & Ozanne, 2015). This is significant considering that the levels of these hormones are altered by obesity – and therefore also during an obese pregnancy – providing a potential mechanism by which maternal obesity can directly impact on hypothalamic development in the fetus.
Maternal obesity can also alter offspring dietary preferences. A recent study in non- human primates has shown that maternal obesity greatly increases the preference for fatty and sugary – rather than low-calorie food – in offspring, leading to obesity (Rivera et al., 2015). This is particularly relevant when considering the ease of availability of highly palatable fat- and sugar- rich foods in modern society, and explains why animal models repeatedly report that the offspring of obese mothers are quicker to develop diet-induced obesity when fed a high-calorie diet compared to control animals. The effect of the early life environment on susceptibility to diet-induced obesity may explain why there is variation in risk of obesity in two individuals living in the same obesogenic environment.
Dysfunction in the hypothalamus could also be a cause of disrupted glucose homeostasis in individuals exposed to maternal obesity, as changes to the levels of metabolic hormones such as leptin and insulin during fetal life result in abnormal development of the circuits linking the brain to the pancreas and liver, which are important for maintaining glucose homeostasis. Due to the importance of the hypothalamus – pituitary – adrenal axis in regulating stress responses, changes in the hypothalamus may also underlie the anxiety phenotypes often reported in offspring in rodent and NHP models of maternal obesity.
The offspring of obese mothers do not only have an increased risk of becoming obese, but also of other related disorders such as cardiovascular disease. Evidence from the Helsinki birth cohort has demonstrated a positive association between maternal obesity and offspring cardiovascular disease and type 2 diabetes. Similarly in the sibling pair studies mentioned earlier, the sibling exposed to maternal obesity has a higher blood pressure compared to their un-exposed sibling (Guenard et al., 2013). Causative associations between maternal nutrition and offspring cardiovascular function have been demonstrated in animal models, which have shown striking evidence of cardiac structural and functional changes. Such changes are often reported prior to changes in offspring body weight, demonstrating that cardiovascular dysfunction is programmed independently of body weight phenotypes. The consequences of maternal-obesity-induced alterations in cardiac structure (and thus function) are clear: a study of a human cohort by Reynolds et al. showed that offspring of obese mothers have a decreased life expectancy due primarily to cardiac dysfunction (Reynolds et al., 2013).
Molecular mechanisms underlying phenotypes in the offspring of obese mothers
Whist the phenotypes associated with exposure to maternal obesity are relatively well described, the underlying mechanisms and the factors related to an obese pregnancy that transmit the effects from mother to fetus remain unclear. It has recently been reported that some metabolic phenotypes arising as a result of maternal obesity in animal models can be transmitted trans-generationally to the grand- and even great-grand- offspring of obese mothers. This, combined with the stable nature of cardio and metabolic phenotypes throughout the lifetime of the exposed individual, suggests permanent changes in gene expression. Epigenetic regulation represents a stable but modifiable level of genomic regulation; the term epigenetics literally means ‘on top of genetics’ and refers to a system of processes that induce heritable changes in gene expression without altering the genomic sequence. Significantly, in vitro experiments have shown that the activity of much of the epigenetic machinery is dependent on energy availability, and could therefore be altered in situations of nutrient excess. There is emerging evidence from human studies of the impact of maternal obesity on the offspring epigenome. A study of siblings with different exposures to maternal obesity in utero showed significant differences in the methylation and expression of gluco-regulatory genes (Guenard et al., 2013). Many studies in animal models are now aiming to establish whether the disease phenotypes previously reported are caused by – rather than associated with – epigenetic changes.
A role for accelerated ageing?
As humans undergo the natural ageing process they display increased body weight, a shift in adipose distribution, and deteriorating function of organs such as the heart, kidney and reproductive system. Many of these natural ageing processes are the same phenotypes observed in offspring exposed to maternal obesity in utero. This has led researchers to consider whether accelerated ageing is one of the mechanisms underpinning the phenotypes related to exposure to an adverse early life environment. Indeed, several animal studies have shown that under-nutrition in utero causes accelerated cellular ageing in organs including the pancreas, liver and skeletal muscle.
Telomeres are guanine-rich nucleotide sequences present at the ends of chromosomes that prevent chromosomal deterioration. An essential part of the ageing process in telomerase-negative somatic cells is telomere shortening that occurs after each cell division. When telomeres become critically short in length, they undergo a conformational change which results in them representing double-stranded breaks, causing the cell to enter growth arrest and senesce or become apoptotic. Differences in telomere length have been implicated in developmental programming in response to maternal body weight. A recent study has shown that maternal pre-pregnancy BMI is negatively associated with telomere length in both the placenta and cord blood at birth (Martens et al., 2016). If similar changes are present in other tissues they could explain the organ dysfunction that ultimately develops in the offspring of obese mothers, and suggest this phenotype is caused by an accelerated ageing process.
Breaking the cycle of obesity transmission
The current research from animal models suggests that the majority of phenotypes in offspring exposed to maternal obesity are caused by either structural changes in whole organs or gene expression changes at a cellular level. In both instances, these changes are likely mediated by altered hormone and nutrient levels resulting from the maternal obesity (acting directly or via intermediary mechanisms such as oxidative stress). Some of the current trials underway in the UK include studies aiming to normalise metabolic hormone levels in obese mothers via lifestyle intervention (in the form of increased exercise), or pharmacological intervention with insulin-sensitising drugs. In some instances, if the intervention is found to correct a defined aspect of the maternal physiology the study can also be used to investigate the importance of this specific factor in mediating the detrimental effects on offspring health. This is particularly true when an intervention can be modelled in animal as well as human studies, as animal studies allow us to examine at a molecular level what effect the intervention is having on the mother. These investigations will be instrumental in allowing us to discover the ‘programming factor(s)’ in the mother that are responsible for causing the offspring phenotypes. This is vital in achieving the ultimate aim of developing future clinical advice and treatment for obese mothers during pregnancy that will stop the transmission of obesity risk to the offspring.
References
Dearden L & Ozanne SE (2015). Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol 39, 3-16
Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, & Vohl MC (2013). Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci USA 110(28), 11439-44Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot
TS (2016). Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med 14(1), 148
Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al (2013). Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347, f4539
Rivera HM, Kievit P, Kirigiti MA, Bauman LA, Baquero K, Blundell P, et al (2015). Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring.Obesity (Silver Spring) 23(11), 2157-64
