Physiology News Magazine
Physiologists, watch where you place that cannula!
Physiologists often sample blood during their research, but are we guilty of failing to question why we draw blood from a given vein? The most appropriate site to sample blood from will depend primarily on the study design and outcome measures, and this decision can influence the interpretation of the data generated.
Features
Physiologists, watch where you place that cannula!
Physiologists often sample blood during their research, but are we guilty of failing to question why we draw blood from a given vein? The most appropriate site to sample blood from will depend primarily on the study design and outcome measures, and this decision can influence the interpretation of the data generated.
Features
Rob Edinburgh, James Betts, Dylan Thompson & Javier Gonzalez
Department for Health, University of Bath, UK
https://doi.org/10.36866/pn.109.20
It is common in physiology research to sample blood for the assessment of the concentration and/or activity of an array of metabolites (e.g. glucose), hormones (e.g. insulin), enzymes (e.g. creatine kinase) or specific cell types (e.g. immune cells). These measures are also taken under a wide variety of conditions such as in the fasted versus fed state, or after rest versus after exercise. However, we often come across research studies asking similar questions, but where different blood sampling methodologies have been used. This can not only influence the concentrations of the measured metabolite or hormone, but in instances where different sampling methods are used, also make comparisons between studies more challenging. When designing research, blood sampling methods are a key consideration and physiologists should always ‘watch where they place their cannula!’

As physiologists, we regularly measure metabolites (e.g. glucose, fatty acids) or hormones (e.g. insulin) in blood samples, sometimes when our participants are fasted, and/or after a physiological challenge, such as the oral glucose tolerance test (OGTT) or a more appetising mixed macronutrient meal (such as a milkshake!). However, when comparing studies that attempt to answer similar questions, it is not uncommon to find that blood might be sampled from an antecubital (elbow) vein, a heated hand vein, interstitial fluid, a finger, or even the earlobe! Therefore, the key question to ask is, does the blood sampling site matter?
We spend a lot of time perfecting pipetting skills to ensure that at the measurement stage our results are accurate, and go to great lengths to make sure that blood samples are collected at specific time points, depending on the outcome measures of the research. Even before we get started with data collection, many hours are spent refining study designs. However, do we always give sufficient attention to the most appropriate blood sampling methods? How does this decision influence the measured concentrations of metabolites or hormones? How can we compare data from studies where different sampling methods are used? These are questions which may sometimes be overlooked.
The worrying prevalence and current trends regarding type 2 diabetes are well documented, and we have also known for a long time that blood glucose levels after a meal can predict risk of metabolic disease. As a consequence, glucose tolerance and indices of insulin sensitivity are frequently used in research to assess disease risk and responses to interventions. These measures will therefore be used as our example for exploring the importance of sampling methods.

Insulin-resistance in skeletal muscle is one of the first defects in the onset of metabolic disease. Whilst arteries are the preferred site for determining peripheral (e.g. muscle) exposure to metabolites such as glucose, or hormones such as insulin (Figure 1), antecubital veins are a more common blood sample site, partly due to the increased risks associated with arterial cannulation, such as greater bleeding from the sampling (puncture) site and blood clots. These risks also make investigating artery-venous differences across a tissue a more difficult procedure.
However, heating the hand to around 37°C causes our capillaries to vasodilate and disperse heat, and since the 1920s we have had evidence that this produces venous blood samples that have a similar oxygen and carbon dioxide composition to arterial blood (Goldschmidt and Light, 1925). These samples are often referred to as arterialised or arterialised-venous blood. It has also been shown that the veins of a heated hand provide concentrations of many metabolites including glucose, fatty acids and amino acids as well as hormones including insulin, that are consistent with concentrations measured in arteries. Even increasing the air temperature of a room can increase venous concentrations of glucose and insulin.
Although some studies use the heated-hand technique to provide arterialised blood for measures of glucose tolerance, many derive these outcomes from non-arterialised venous blood, interstitial fluid or finger prick samples. This is perhaps because there is no clear consensus or justification in the literature of the most appropriate blood sampling method for an OGTT (or meal challenges).
The chosen blood sample method may be determined by availability of equipment or specialist staff and the measurement period. For example, finger prick samples can be more easily done in a field setting and without the need for cannulation or venepuncture. However, sometimes an explanation for the chosen method is not clear and one method (e.g. antecubital venous cannulation) is chosen over another (e.g. the heated hand technique), when either could have been implemented.
This inconsistency raises important questions:
i. How does the sample method influence the concentration of the metabolite/hormone being measured?
ii. How easy is it to compare between studies where different sampling methodologies are used?
If we remind ourselves of some basic physiology, it is unsurprising that the sample method can alter the measured concentrations of many metabolites and hormones. To better demonstrate this, we will continue with our example for glucose, but this principle applies to many constituents of blood.
If humans are fasted, blood glucose concentrations are maintained at around 4 to 5 mmol/L, with the extracted glucose (primarily by the brain, muscle and other tissues) mostly replaced by the liver. Upon ingesting carbohydrates, blood glucose concentrations rise. Insulin is then produced to lower blood glucose concentrations by supressing glucose appearance from the liver and increasing glucose clearance into (mostly) skeletal muscle. The glucose and insulin are transported to the peripheral tissues via arteries where they interact with the tissues, with insulin binding to its receptors to trigger a process which results in greater glucose transport into insulin-sensitive cells. Veins then carry the blood back to the lungs after this interaction has taken place.
As a consequence, concentrations of glucose and insulin are typically higher when measured in arterial (or arterialised) versus venous blood due to the removal of glucose for metabolism and/or storage in peripheral tissues, and some binding of insulin to the cell membranes. For similar reasons, glucose concentrations are higher in capillary plasma (finger prick or earlobe samples) versus veins (Figure 1).
Continuous monitoring of glucose over a longer time (weeks) is increasingly common, typically in interstitial fluid of adipose tissue. This allows for assessment of patterns of glucose levels in a free-living setting. However, plasma and interstitial fluid are distinct compartments, with glucose transferred from capillaries to the interstitial fluid by movement across the endothelial cell layer of blood vessels at a rate primarily determined by blood flow (Cengiz and Tamborlane, 2009). Under steady-state conditions for glucose (fasting and rest), interstitial fluid and plasma are reasonably well matched. However, under non-steady state conditions, and when blood flow is altered, differences between methods can become increasingly apparent. Moreover, across certain populations, blood flow to adipose tissue may be different (e.g. obese versus non-obese individuals), which may also influence any variation between concentrations of metabolites in blood versus interstitial fluid.
This brings us to a second and more important consideration. If differences between blood sample methods were similar under all conditions (e.g. rest versus exercise or fasting versus after a meal) and populations (e.g. people with versus without diabetes), simple correction factors could be applied. This would allow for easier comparisons between studies that have investigated similar questions using different sample methods or conditions (e.g. rest versus exercise) within a study.
However, this is not the case. Whilst concentrations of metabolites or hormones are normally similar across any artery in the body at a given time, venous concentrations can differ due to a net uptake or release from the tissue beds in proximity to the sampling site. For example, exercise increases insulin sensitivity and glucose uptake in exercised muscle and can impair insulin sensitivity in non-exercised muscle. As such, the difference in metabolite levels in arterialised versus venous blood will depend on the nutritional and metabolic status (e.g. rested versus exercised) of an individual.
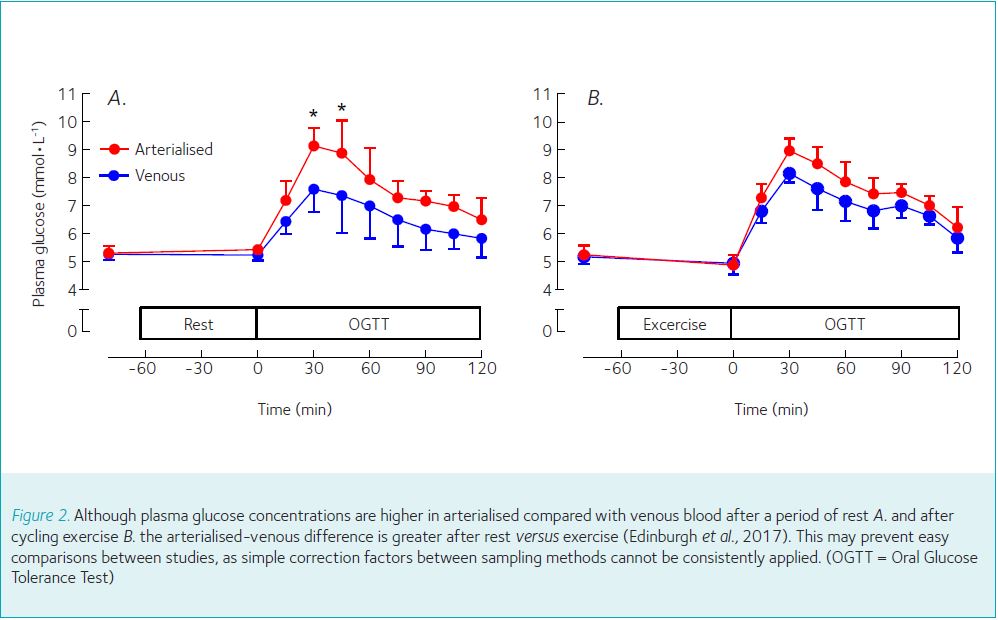
Some guidelines provide corrections for venous to capillary plasma for an OGTT, but only at rest (e.g. + 1.1 mmol/L; [WHO, 1985]). However, the difference between arterial and venous levels for glucose is higher in the fed versus fasted state (Glassberg, 1930). The variation in glucose concentrations between venous and arterialised samples is also different after exercise versus rest (Figure 2, Edinburgh et al., 2017). Therefore, it appears that simple corrections between sample methods cannot consistently be applied in all scenarios.
The determining factor here is the interaction of the periphery with the blood. When comparing samples collected after a meal (or during a hyperinsulinaemic clamp) versus fasting, the arterialised-venous difference for glucose will be larger due to the higher net extraction of glucose from the blood. A similar finding is apparent after exercise, although this will depend on whether the vein used for sampling is draining exercised or non-exercised muscle. The population studied is important, with different arterialised-venous differences detected in people with versus without diabetes
(Rabinowitch, 1927), likely due to differences in the efficiency of glucose disposal. It should also be mentioned that concentrations of metabolites may not always be higher in arterialised versus venous blood. If peripheral tissues are producing a metabolite at a rate greater than the extraction from the blood (e.g. lactate from muscle during/after exercise or leptin from adipose tissue) then venous concentrations may be higher than those measured in arterialised blood. The magnitude of this difference will again depend on the conditions in which a sample is collected.
Implications
Given that there are a number of different metabolites/hormones that are routinely measured, and across many different conditions (resting, after a meal, after exercise and so on), how can we develop correction factors for each possible scenario? This is not very practical, so instead we suggest two key points regarding blood sampling methods:
1.Think carefully about the main outcome measure and this will inform which sample method is most appropriate.
The blood sampling method chosen will impact upon the measured concentrations of metabolites and hormones in blood. The most appropriate method for a given study will depend on the outcome measure. For an example of glucose,if you are interested in the exposure of peripheral tissues to circulating concentrations then arterialised blood may be preferable. This is also the preferred method for studying the rate of blood glucose appearance using tracers. However, venous blood may give a better indication of metabolites or hormones released from specific muscles and adipose tissue depots. Continuous glucose monitoring provides useful data, but should be interpreted in the context of the population studied, and conditions in which samples were collected.
2. Always report the sample method used as this may influence the interpretation of the data, especially when comparing studies.
Conclusions
Physiology research often involves blood sampling, but a number of different methodologies exist. Decisions made regarding the sampling methods can influence the measured concentrations of metabolites, hormones, enzymes and cell types. The key factor is the interaction of peripheral tissues with the blood and whether there is a net extraction, release or metabolism of a metabolite, hormone, enzyme or cell. This in turn will depend on the population studied and the conditions in which the blood was sampled. Thus, physiologists should always (1) think about the tissues and analytes of interest, (2) report and justify sampling methods and (3) watch where they place their cannula!
References
Cengiz E & Tamborlane WV (2009). A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technology & Therapeutics 11(S1), S-11-16.
Edinburgh R et al. (2017). Prior exercise alters the difference between arterialised and venous glycaemia: implications for blood sampling procedures. British Journal of Nutrition, 117(10), 1414-1421.
Glassberg B (1930). The arteriovenous difference in blood sugar content. Archives of Internal Medicine 46(4), 605–609.
Goldschmidt S & Light AB (1925). A method of obtaining from veins blood similar to arterial blood in gaseous content. Journal of Biological Chemistry 64(1), 53–58.
Rabinowitch I (1927). Simultaneous determinations of arterial and venous blood-sugars in diabetic individuals. British Journal of Experimental Pathology 8(1), 76-84.
World Health Organization (1985). Diabetes Mellitus: Report of a WHO Study Group [meeting held in Geneva from 11 to 16 February 1985]. http://apps.who.int/iris/bitstream/10665/39592/1/ WHO_TRS_727.pdf