
Physiology News Magazine
Why are we still using animals to study human neurophysiology?
Could stem cells completely replace animal models in research one day?
Features
Why are we still using animals to study human neurophysiology?
Could stem cells completely replace animal models in research one day?
Features
Eric Hill
Life and Health Sciences, Aston University, UK
https://doi.org/10.36866/pn.101.30

Animal models have been central to our understanding of human physiology, but human stem cell based models may help us to further our knowledge and tackle devastating diseases such as Alzheimer’s disease. Stem cell-derived brain cells have emerged as a powerful tool to model neuronal behaviour and disease pathology; their increasing use in drug discovery promises to help speed up drug screening and reduce the number of animals used at the earliest stages of testing. Here I discuss the utility of these cells, potential pitfalls and recent developments in the field.
A scarce resource
Logically, human tissue would be the best platform for the study of human diseases, although for obvious reasons, such tissue is not always readily accessible. This is particularly apparent for the study of the human central nervous system (CNS). Consequently, animals as diverse as worms, flies and various mammals are currently used to model human physiology and neurophysiology.
These systems have given us a great deal of information regarding our normal biological processes, but some aspects of our physiology and neurophysiology are uniquely ‘human’. This is borne out by difficulties in reproducing many diseases in animals that affect significant numbers of the elderly, such as Alzheimer’s disease. Indeed, not even the latest and most sophisticated transgenic models recapitulate all of the features of this complex neurodegenerative condition. Clearly, such conditions require highly specialised and relevant experimental platforms for us to even begin to understand their complex aetiology. Compellingly, in the public as well as the medical and scientific minds, stem cell technology now has a powerful appeal in the search for such platforms.
The potential of stem cells
Stem cells are unspecialised cells that are capable of self-renewal and are able, under the correct conditions, to differentiate into all of the different cells within the body. Since the derivation of the first human embryonic stem cell line (hESCs) in 1998, the field of stem cell biology has rapidly expanded. Furthermore, the development of induced pluripotent stem cells (iPSCs) has further increased the potential use of stem cells whilst avoiding ethical issues associated with the use of embryos. This revolutionary technique allows scientists to produce stem cells from the skin cells of patients thus providing highly relevant patient/genotype specific models in which scientists can study disease processes.
Stem cells have now been used to produce a variety of different cell types and tissues such as brain, heart and gut. For many scientists, the production of neurons has been of great interest as it allows researchers access to human brain cells. More importantly, the production of specific populations such as cortical or dopaminergic neurons has allowed researchers to investigate the activity of neural networks from particular regions of the brain. As such, the potential to produce human brain cells from sources such as stem cells has huge potential for studying human disease, as well as providing tools for drug screening.
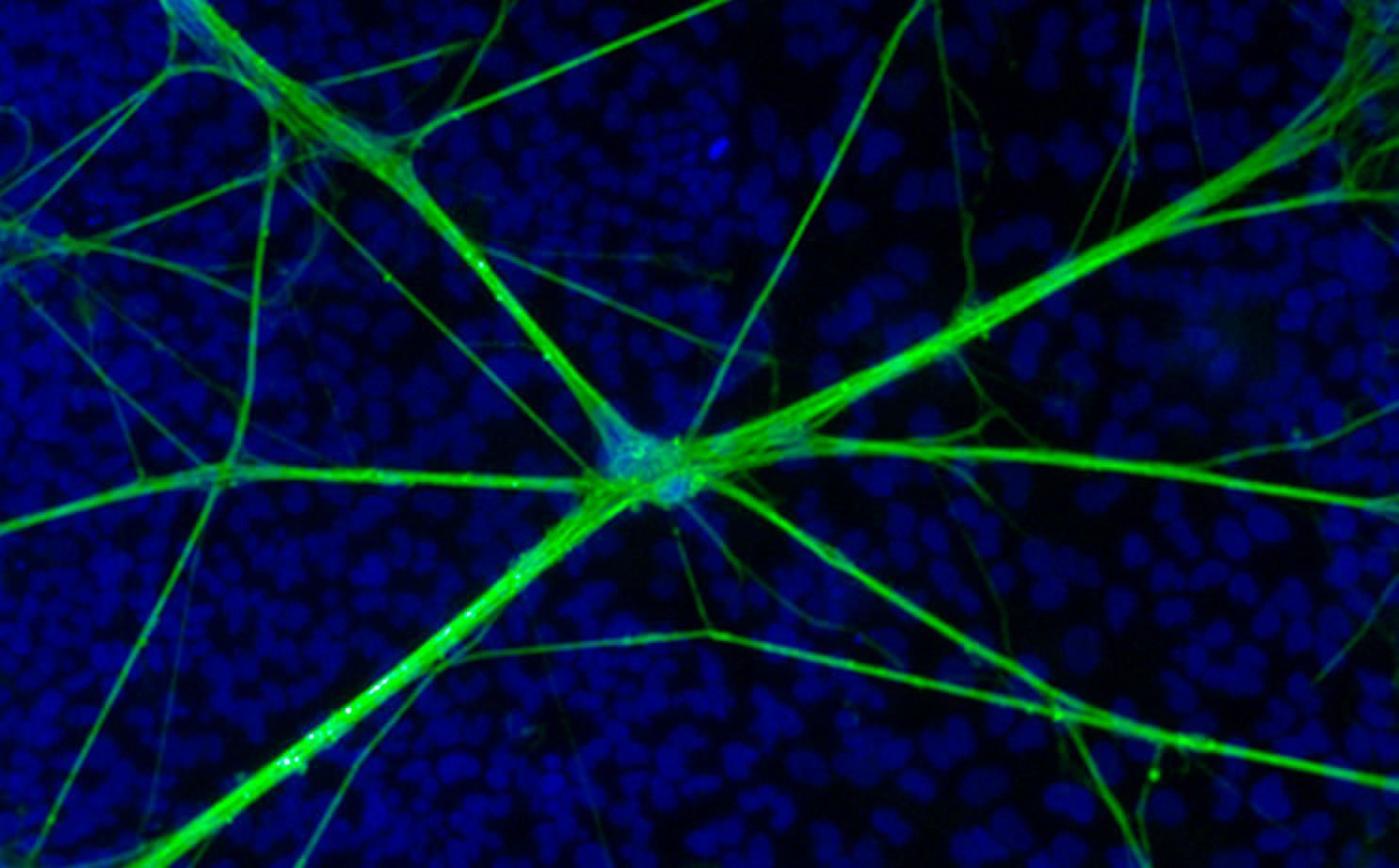
Are these cells physiologically comparable to animal or even actual human brain cells?
Understandably, there is great excitement surrounding the development of iPSC technology particularly in the area of disease modelling. It is also undeniable that public expectation is also significant, especially in the areas of much-feared and currently intractable neurodegenerative conditions such as Alzheimer’s’ and Parkinson’s diseases. However, it remains to be seen whether these cultures can actually recapitulate many of the processes observed in animals or human derived cells. Whilst some studies have only described the presence of neuronal markers and cell morphology, the description of the hallmark features of genuine neurons such as the ability to fire action potentials is now commonplace. Using both human embryonic stem cells and induced pluripotent stem cells researchers have described the development of neuronal features including synaptic activity, neurotransmitter release, network formation, highlighting the maturation of these cells. However, currently no studies have reported synaptic plasticity, a key feature of CNS learning and information processing, in stem cell derived neurons.
Stem cell modelling like much of neurophysiology has focused on neurons
Unfortunately, many of the studies that have developed protocols for the derivation of neural cells have not considered the importance of glial cells such as astrocytes. Astrocytes are no longer viewed as providing a structural role within the brain and are now known to be intimately involved with neuronal signalling, with astrocytes and neurons forming what is now termed the ‘tripartite synapse’ (Volterra and Meldolesi, 2005). Furthermore, astrocytes represent a complex and diverse network of cells that plays a role in information processing and behaviour. The study of human neuron and astrocyte cultures is potentially more complex than other cell types, since we do not really know or understand how human neuron and astrocytes
function to the same extent, as we understand rat model neurons. Human astrocytes are much larger and more functionally diverse than their rodent counterparts (Oberheim et al., 2009) and it has even been suggested that human intelligence may in part be due to the actions of glial cells. Human astrocytes (glia) have evolved to be more complex than rodent astrocytes and thus, must contribute to specific human brain functions. Such findings indicate significant and important roles for human astrocytes, which support the notion that these cells should be given equal importance to that of neurons in the development of stem cell derived neuronal networks.
The ‘star’ of the show
We have previously shown that stem cell-derived astrocytes form an interconnected network that allows the propagation of calcium waves between cells via gap junctions. These astrocytes respond to neuronal activity and also communicate with each other via the release of gliotransmitters (Hill et al., 2012). More recently we have demonstrated that these cells alter their metabolism in response to neuronal activity, a process which has previously only been observed in rodent models (Tarczyluk et al., 2013). A number of labs have now developed efficient protocols to produce highly enriched cultures of astrocytes from hESCs and iPSCs that display the features of mature astrocytes (Shaltouki et al., 2013). It is now clear that if new cellular platforms are intended to generate functional neuronal networks that behave as networks do in the brain, then all the cellular components that comprise those networks, such as astrocytes, must be included in a viable model. Indeed, studies have shown that human neural precursor cells (hNPCs) grown in the presence of astrocytes mature at a faster rate than neurons alone. The presence of astrocytes also enhances the survival of cells; in our laboratory co-cultures containing astrocytes extend neuronal survival four-fold, to beyond twelve months.
What’s my age again?
A limiting factor in the use of iPSC-derived neurons to model age related disease is the biological age of cells. iPSC-derived neurons mature over a period of months. However, even at this stage, cells may resemble tissues derived from 8–10 week old foetuses (Mariani et al., 2012). This foetal-like nature is clearly an issue for investigators hoping to study late onset diseases such as Alzheimer’s. However, a number of strategies to expedite the ageing of cells in culture have been employed (Miller et al., 2013) with the aim of recreating cell types of specific ages.
The third dimension
Traditional 2D in vitro models lack the structural organisation required to recapitulate the complex networks observed in the brain. A key developmental event such as the formation of cortical layers is not observed in 2D culture. However, the rapid expansion of organoid culture protocols have led to the conditions that, to a high degree, reproduce the in vivo environment and allow the production of defined cortical layers (Lancaster et al., 2013). This intriguing technique has huge potential for studying human development as well as a range of neurological disorders.
The future
Satisfying the powerful public, scientific and medical expectations of stem cell technology, with regard to human CNS study and therapy is a daunting challenge. The models must be relevant, reproducible, practical, yet sufficiently flexible to undergo cross-adaptation to a variety of different purposes, ranging from basic research in CNS development and function, through to drug efficacy and toxicity studies. Perhaps the key to the success of iPSC derived models is their ability to produce neuronal networks that acquire mature circuitry capable of complex tasks such as memory formation. Such a feature is the cornerstone of relevance to man. Indeed, the ability to generate specific cell types as well as 3D structures will offer alternative culture techniques that will enhance these functions. The combined use of gene editing techniques will enable researchers to manipulate stem cell platforms to study CNS diseases in ever greater detail. Judging by the pace of recent progress, it is perhaps only a matter of time before human stem cell-derived models will reproduce the actual network connectivity and functions that are observed in our own brains so that they will offer an attractive and compelling alternative to the use of animals in studying human physiology.
References
Hill EJ, Jimenez-Gonzalez C, Tarczyluk M, Nagel DA, Coleman MD & Parri HR (2012). NT2 derived neuronal and astrocytic network signalling. PLoS One 7, e36098
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP & Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373-9
Mariani J, Simonini MV, Palejev D. Tomasini L, Coppola G, Szekely AM, Horvath TL & Vaccarino FM (2012). Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA 109, 12770-5
Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner T A, Rossi DJ & Studer L (2013). Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13, 691-705
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann J G, Ransom BR, Goldman SA & Nedergaard M (2009). Uniquely hominid features of adult human astrocytes. J Neurosci 29, 3276-87
Shaltouki A, Peng,J, Liu Q, Rao M S & Zeng X (2013). Efficient generation of astrocytes from human pluripotent stem cells in defined conditions.
Stem Cells 31, 941-52
Tarczyluk MA, Nagel DA, O’neil JD, Parri HR, Tse EH, Coleman MD & Hill EJ (2013). Functional astrocyte-neuron lactate shuttle in a human stem cell-derived neuronal network. J Cereb Blood Flow Metab.
Volterra A & Meldolesi J (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6, 626-40
