
Physiology News Magazine
Are medicinal leeches still a useful model for studying neurophysiology?
From medical tool to model organism
Features
Are medicinal leeches still a useful model for studying neurophysiology?
From medical tool to model organism
Features
Joshua Puhl
Section on Developmental Neurobiology, NINDS, Bethesda, USA
https://doi.org/10.36866/pn.99.24
A question I am frequently asked, as an investigator who works on leeches, is ‘can further studies of these simpler animals generate sufficiently interesting information to justify their continued study?’ The answer is ‘yes’, studying the CNS of leeches can still make a useful and relevant contribution to modern neuroscience research.

The medicinal leech has several advantages for studying many general principles of nervous system function. These include the organization of neural networks that generate behaviors and the mechanisms of decision-making used to select appropriate behaviors. Historically, the European medicinal leech (which I will refer to as the leech in this article, for the sake of brevity; species: Hirudo medicinalis and Hirudo verbena, see Fig 1) has been studied to understand better the properties of mechanosensory neurons, motoneurons, synaptic communication as well as the organization of neural circuits capable of generating behaviors (Reviewed by: Kristan et al. 2005). More recently, some researchers have given less attention to model systems like the medicinal leech in favor of more complex and human-like animal models such as rodents and zebrafish. I will explain the continued value of the leech by briefly describing some of the features of its central nervous system (CNS) that make this animal well-suited for studies of the neural mechanisms of behavior. I will also give some examples of recent work done to understand better the generation and control of behavior at the levels of neural networks and individual neurons.
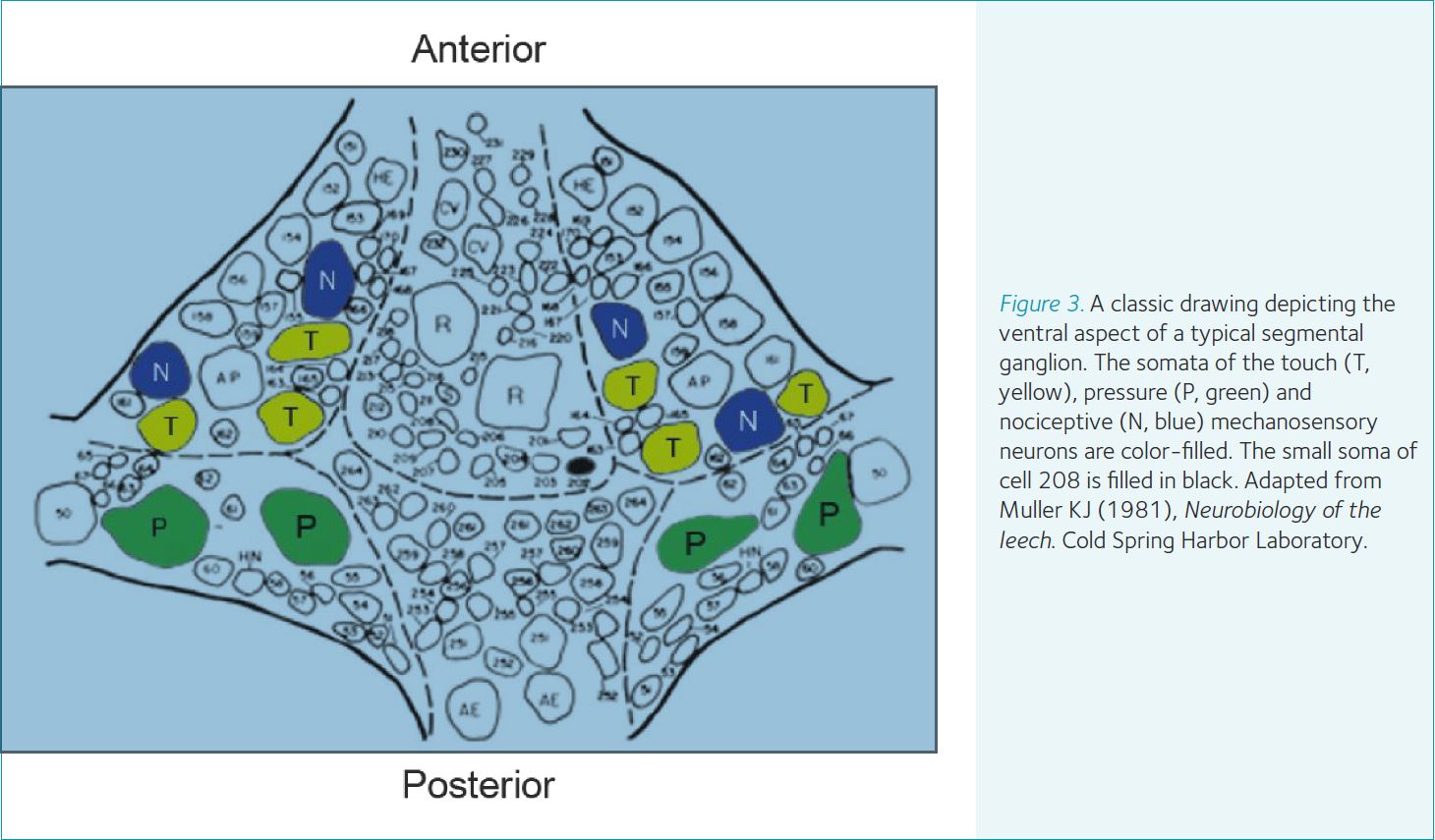
The CNS and behavioral repertoire of leeches have features that facilitate addressing many important questions in neuroscience. The ventral nerve cord (i.e., the CNS) of the leech contains approximately 10,000 neurons divided between a compound cephalic ganglion, 21 segmental ganglia and a compound tail ganglion (Fig. 2). Each segmental ganglion contains approximately 350-400 neurons or about 200 bi-lateral pairs. Compared with the CNS of insects and vertebrates, which have millions or billions of neurons within their CNS, leeches are a potentially more tractable system. This is especially true when the research goals are to understand the contributions of individual neurons in generating a behavior, which is often among the goals of studies involving leeches. One of the principle features making these types of studies possible is that single neurons in the CNS are uniquely identifiable between individuals based on the size and location of the neuron’s soma, its electrophysiological signature, neurotransmitter phenotype, connectivity with other neurons and its morphology (Fig. 3). Furthermore, the CNS, which resides inside of the animal’s circulatory system, lacks a formidable blood-brain barrier. This fact makes delivery into the CNS of many neuroactive substances, in vitro and in vivo, all but trivial in most cases. Lastly, although the CNS of these animals contains far fewer neurons compared to insects and vertebrates, leeches exhibit an impressive array of dynamic and adaptable behaviors common to nearly all animals. These behaviors include (but are not limited to) hunting and searching, feeding, mating as well as two primary forms of locomotion, crawling and swimming.


These features, along with over 75 years of research, have established a solid base from which to ask questions relevant to contemporary thinking on the neural mechanisms of behaviors. For example, the locomotor behaviors have been well studied at the level of identified neurons and the role of many such neurons has been established in the generation of these behaviors. These studies include cells located within the segmental ganglia that generate timing and motor commands, as well as descending cephalic neurons which act in a command or executive capacity to control the segmental neural networks. Work done on swimming and crawling has established that the neural circuits within the segmental ganglia underlying these behaviors, called central pattern generators, have overlapping and multifunctional elements. Many of the identified descending cephalic neurons can control and modulate both behaviors. Recent work has established that the decision to swim or crawl is influenced by a number of factors. Briggman et al. (2005) showed that during the decision-making period just prior to activation of locomotion the activity level of a single identified neuron (named cell 208; shaded black in Fig. 3) biased the CNS to produce one form of locomotion over the other. Biogenic amine modulators like dopamine and serotonin, which act at both the segmental and cephalic levels, predictably influenced the type of locomotion expressed in isolated CNS preparations. Finally, signaling from a multifunctional identified descending cephalic neuron onto cells housed in the segmental ganglia (named R3b-1) is both necessary and sufficient for crawling but only activates and modulates swimming (it is not necessary for swimming; Puhl et al. 2012). These results and others like them have inspired new projects where investigators are probing how higher-order, decision-making cephalic neurons interact with segmental locomotor circuits as well as how neuromodulators influence their interactions. These new projects aim to elucidate how higher-order ‘command’ systems control multifunctional central pattern generators, at a level of detail focused upon individual neurons. This would be a difficult or impossible task in nearly all currently studied organisms, but is likely to be a tractable one using leeches.
In addition to locomotion, recent studies of leeches have contributed to other areas of active investigation in neurophysiology. Engineers have enlisted the help of those who study medicinal leeches to assist in the development of new technologies for physiology research. For example, leech CNS preparations were employed to test and validate voltage-sensitive dye imaging (Miller et al. 2012) and nanowire intracellular electrode fabrication methods (Ferguson et al. 2012). These technologies are being adapted for use in other animal models.
Leeches also possess cells with interesting electrophysiological properties worthy of study in the context of the neural mechanisms of behavior. One such neuron is the segmental non-spiking (NS) neuron which does not generate normal sodium-dependent action potentials, but does communicate with many other cells within a segmental ganglion (mostly motoneurons). Researchers at the University of Buenos Aires recently found that these NS neurons modulate the activity of motoneurons during locomotion and may help to regulate the electrical properties of these cells during ongoing behavioral expression and switching between behaviors. Their current work is probing these very ideas.
A team at Emory University is looking at the variability in the strength of synapses among identified neurons and how this variability affects the function of neural circuits such as the well-characterized leech heart central pattern generator. They found that, between individual leeches, there is a great deal of variability in the synaptic coupling between the identified neurons of the heart central pattern generator. In the face of this variability this neural circuit is still able to generate predictable and stereotypical outputs. Using a combination of ‘wet’ biological and computational modeling techniques these researchers and their collaborators are beginning to describe, in detail, the mechanisms which underlie these observations. Along this same line of inquiry, another recent report from a lab at the University of Minnesota, Twin Cities established that acute removal of descending cephalic signaling, via transection of the ventral nerve cord just below the cephalic ganglion, led to the degradation of crawling in intact leeches. Curiously, without restoration of the damaged neural fibers, the disrupted crawling behavior spontaneously returned days to weeks after chronic removal of these descending signals. These results indicate that the crawl-generating neural networks were able to adapt to restore function without the cephalic inputs normally required for crawling. Presumably, this adaptation arises through changes in the cell-to-cell signaling or in the electrical properties of crawl-generating neurons. Follow-up work may lead to new insights into mechanisms of how neural circuits adapt to restore behavioral function after catastrophic injury.
The last example I will share is work done by a group at the University of California at San Diego who studied competitive behavioral selection of the leech feeding behavior. Their paper proposed a mechanism for the preferential selection of feeding over other behaviors via modulation of the synaptic strengths from sensory cells onto premotor neurons. They determined that this modulation was mediated by serotonin.
On top of their many contributions to scientific research, leeches are often used to help train new scientists; a task that should never be taken lightly. Because of their accessibility features these animals are well suited for use in behavior and basic neurophysiology teaching labs. Often, with some determination and a little luck advanced undergraduate and graduate students can perform their first independent recordings of identified neurons in just a day or two. Within a relatively short time period, these students can design and perform sophisticated experiments that confront ideas in neurophysiology and the neural bases of behavior (with a bit of guidance from their mentors, of course). A well-established example which supports this assertion comes from the Neural Systems and Behavior course offered each summer at the Marine Biological Laboratories in Woods Hole, MA, USA (http://www.mbl.edu/education/summer-courses/neural-systems-behavior/). Leeches can be even more useful when engaging inexperienced undergraduate and high school students. Using hands-on demonstrations and lab exercises, they can see concepts they learned in lectures and classroom discussions come alive right in front of them. In many cases, these students can participate in novel neuroscience research during laboratory exercises without the months of training required to do so in more complex models.
In this short article I have described some of the features of medicinal leeches that make these simpler animals useful for addressing important questions in neurophysiology along with providing some examples of current research which exploit these features to elucidate the mechanisms underlying dynamic and adaptive behaviors common to nearly all animals. Although I was not able to mention every significant study using these animals, my hope is that I have inspired some readers to do some follow-up reading. I have provided some interesting examples that establish that the CNS of these animals is a relevant and useful model for studying the neural bases of behaviors and behavioral selection in the context of modern neurophysiology teaching and research.
References
Briggman KL, Abarbanel HDI & Kristan WB Jr. (2005). Optical imaging of neuronal populations during decision-making. Science 307, 896–901
Ferguson JE, Boldt C, Puhl JG, Stigen TW, Jackson JC, Crisp KM, Mesce KA, Netoff TI & Redish AD (2012). Nanowires precisely grown on the ends of microwire electrodes permit the recording of intracellular action potentials within deeper neural structures. Nanomedicine 7, 847–853
Kristan WB Jr, Calabrese RL & Friesen WO (2005). Neuronal control of leech behavior. Prog Neurobiol 76, 279–327
Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB Jr & Tsien RY (2012). Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci USA 109, 2114–2119
Puhl JG, Masino MA & Mesce KA (2012). Necessary, sufficient and permissive: a single locomotor command neuron important for intersegmental coordination. J Neurosci 32, 17646–17657
