
Physiology News Magazine
The mighty protein: insulin-like growth factor type 1
IGF-1 plays a critical role in skeletal muscle growth during development, muscle regeneration and muscle hypertrophy in response to training.
Features
The mighty protein: insulin-like growth factor type 1
IGF-1 plays a critical role in skeletal muscle growth during development, muscle regeneration and muscle hypertrophy in response to training.
Features
Fan Ye & Stephen E Borst
University of Florida, USA
https://doi.org/10.36866/pn.97.30
Muscle atrophy and weakness are common clinical phenomena observed following bed rest, surgery, cast immobilization and injury or disease. The consequences of loss of muscle function are far reaching and include decrease of motor control and overall fitness, development of functional limitations and impairment, and long term disability. The recovery of muscle strength and function following injury or disease is a major challenge in rehabilitation.
The ability to manipulate muscle adaptation and growth has great potential in clinical situations where functional ability and strength are compromised. Administration of growth hormone (GH) has been used successfully in elderly to prevent atrophy during catabolic diseases or following hip fractures (Van der Lely et al. 2000). GH administration in these populations resulted in increased insulin-like growth factor 1 (IGF-1) levels, and increased muscle mass and functional ability. IGF-1 plays a critical role in skeletal muscle growth during development, muscle regeneration and muscle hypertrophy in response to training. Several growth factors have been implicated in directing muscle specific gene expression; however, the hypertrophic effects of growth hormone have been thought to be primarily mediated via IGF-1.
IGF-1
IGF-1 was first identified in 1957. It was known by other names including sulfation factors, non-suppressible insulin-like activity, multiplication stimulating activity and somatomedins. It was initially identified on the basis of three unique properties: its mediation of the skeletal growth-promoting actions of GH, its mitogenic properties, and its mimicry of the actions of insulin. This peptide was isolated in Zurich by Rinderknecht and Humbel on the basis of its insulin-like activity, but was renamed IGF-1 when it became apparent that its growth-promoting properties were more important than their insulin-like activities (Rinderknecht & Humbel, 1978).
The IGF-1 system includes ligand IGF-1, its receptor, IGF-binding proteins (IGFBPs) and IGF proteases. The biological significance of the IGF-1 was most strikingly demonstrated when its expression was ‘knocked out’ by homologous recombination techniques. Virtually every component of the IGF-1 system (the various ligands, receptors and binding proteins) has been knocked out, and the results demonstrate that the IGFs are very important in muscle growth and development. Indeed, a common observation in mouse lines lacking IGF-1 (and/or its receptor) is that embryonic development is impaired but embryos are viable. However, the pups die immediately after birth because they cannot breathe. The central importance of IGFs to muscle development is emphasized by the fact that mice in which expression of myogenin has been knocked out show the same result; i.e. there is essentially no functional muscle development in the absence of either IGF-1 or myogenin. The mice with an inactive IGF-1 gene studied by Powell- Braxton et al. (1993) were ‘characterized by underdevelopment of muscle tissue,’ and a ‘generalized organ hypoplasia… including the muscles’. The essential role of IGFs in cell growth is shown by the report that fibroblasts from IGF-1 receptor knockout mice grew very slowly in culture unless they were transfected with a plasmid expressing the IGF-1 receptor. Using the opposite approach in which transgenic animals overexpressed IGF-1, Mathews et al. (1988) found that muscle and bone growth were increased approximately 30% when circulating levels of IGF-1 were 50% above control values. There was a substantial increase in weights of spleen, pancreas, brain, and kidney and an increase in DNA content of these tissues. All of these observations are consistent with the view that IGF-1 plays an essential role during normal growth and development. In spite of the association of excess IGF-2 with tumours, in general it is clear that IGF-1 plays a major role in development, growth, cell differentiation, and maintenance of skeletal muscles, both in culture and in intact animals. Most, if not all of these actions are mediated by the IGF-1 receptor, and they are strongly modulated by IGF binding proteins.

Viral-mediated IGF-1 gene transfer
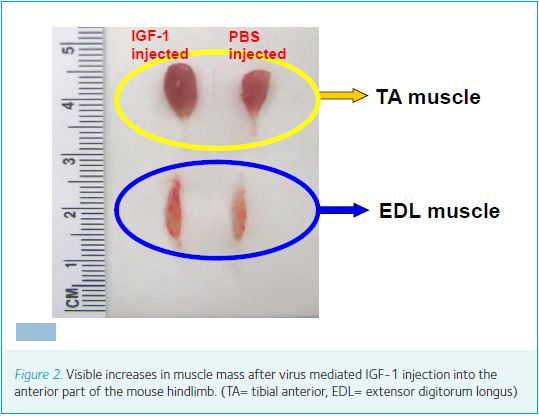
Increasing blood hormone levels may be risky. Specifically, the long-term safety of the activation of GH/IGF-1 levels remains uncertain with regard to tumour growth, as most human solid cancers express IGF-1 receptors. Elevated GH levels have also been linked to impaired glucose tolerance, hypertension and fluid retention. However, given the known autocrine/paracrine actions of IGF-1, local manipulation of IGF-1 expression and secretion by muscle fibres may not only be safer but also more effective (Fig. 1). Recent developments in genetic manipulation are appropriate for the introduction of small regulatory factors, such as IGF-1, into tissue. The recombinant adeno-associated virus (rAAV) vector system consists of rAAV inverted terminal repeats (ITRs) that are necessary and sufficient for replication and packaging of the virus. rAAV lacks virally encoded genes, and therefore can be used to infect adult tissue without rendering an immune response. Viral-mediated gene delivery of IGF-1 has been shown to enable efficient transfer of IGF-1 into both young and adult animals. The rAAV-virus is introduced via direct injection into a muscle compartment and endocytosed by muscle cells (Fig. 2).
IGF-1 and muscle
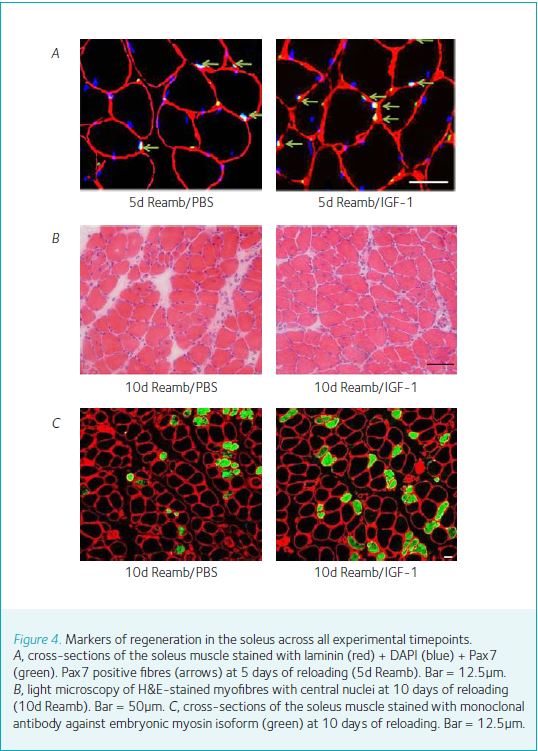
Numerous in vitro studies have shown that exposure of mammalian muscle cells to IGF-1 promotes myogenic proliferation and differentiation as well as protein synthesis. Other studies have shown that administration of IGF-1 induces an increase in muscle protein content and reduces protein degradation. By injecting the gene-virus package into the muscles of adult mice, we (Ye et al. 2013; Stevens-Lapsley et al. 2010) have shown that virally mediated gene transfer of IGF-1 increases muscle size under normal loading/activity conditions (Fig. 3), but that this newly established homeostasis is maintained during cast immobilization, when neuromuscular activity is minimal. Interestingly, despite increased expression of IGF-1, the relative rate of loss of muscle mass and strength is not attenuated in virus-injected versus control muscles. We speculate that the latter is related to a decrease in IGF-1 bioactivity in the absence of neuromuscular activity, due to alterations in receptor density, binding protein, or postreceptor events. Allen et al. (1999) previously postulated that the level of neuromuscular activity affects the expression of the IGF-1 receptor (IGF-1R), making atrophying muscles less susceptible to the effects of endogenous IGF-1. Conversely, during hypertrophy, increased levels of muscle activation and loading may result in greater IGF-1R expression. Finally, we demonstrate that successful gene transfer of IGF-1 increase the muscle’s regenerating capacity. Histological examination of EDL muscles during cast-immobilization and reloading showed an increased number of central nuclei, Pax7 and embryonic myosin in IGF-1 injected muscles during reloading (Fig. 4). The presence of central nuclei and Pax7 in adult myofibres is indicative of recent fusion of activated satellite cells to existing myofibres. IGF-1 has multiple targets in muscle. IGF-1 receptors are found on activated satellite cells, adult myofibres, motor neurons and Schwann cells. Satellite cells, which retain their mitotic capacity, serve as the pool of myonuclei necessary for muscle enlargement. Satellite cells are mobilised in response to increased neuromuscular activity (activity or loading) and injury. Following activation, the satellite cells proliferate, differentiate and fuse with adult myofibres, leading to an increase in fibre size.
One of the more interesting recent developments in the IGF-1 story has been the identification of a unique IGF-1 isoform that is expressed in response to changes in the loading state of skeletal muscles. In both human beings and rats, the IGF-1 gene spans more than 70 kilobases and consists of six exons and at least five introns. Splicing is a complex mechanism by which exons are cut and pasted in different combinations from pre-mRNA. In humans, alternative splicing of IGF-1 pre-mRNA leads to the production of three different transcripts at the 3’ end, resulting in different E-peptides. It is the IGF-1Ec splice variant which has been most closely associated with stretch overload and damage, hence its being termed ‘mechano growth factor’ (MGF). However, using a viral construct and a myosin light chain (LC3f) promoter, Barton et al. (2006) showed that both the MGF and IGF-1Ea gene transfer could increase muscle IGF-1 (mature) expression without increasing circulating IGF-1. It appears that skeletal muscles produce both a generalized tissue-type IGF-1 and the loading-sensitive MGF isoform with differing time courses, suggesting distinct roles for these two growth factor isoforms. Expression of both IGF-1 and MGF appears to be very sensitive to the loading state of the muscle.
IGF-1 and motor neuron
The importance of IGF-1 in motor neuron survival and motor regeneration is quickly emerging. A critical role for IGF-1 in motoneuronal survival has been demonstrated during embryonic and early postnatal life, as well as in spinal cord pathology. In vivo studies show that IGF-1 not only inhibits neuronal cell death, but also stimulates nerve regeneration in crushed or freeze-injured nerves. Rabinovsky et al. (2003) found that after a sciatic nerve crush injury in transgenic mice expressing the human IGF-1 transgene, there is an increase in the number of neurofilaments staining the axons in muscle and an accelerated return of nerve conduction velocity. Similarly, IGF-1 increases intramuscular nerve sprouting 10-fold when administered subcutaneously to normal adult rats (Caroni & Grandes, 1990). Finally, IGF-1 has also been linked to age-related alterations in neuromuscular innervation (Payne et al. 2006). These data indicate that IGF-1 plays an important role in mature motoneuron maintenance, both in the normal state and under conditions where motor neuronal loss is found such as ageing and pathological conditions involving the central nervous system.
It has been shown that rAAV is retrogradely transported from presynaptic terminals of projecting neurons through the entire length of the axon. Kaspar et al. (2003) took advantage of the retrograde transport ability of AAV in a mouse model of amyotrophic lateral sclerosis and injected AAV into respiratory and motor limb muscles to directly target the motoneurons and test the efficacy of two neurotrophic factors, IGF-1 and glial cell line-derived neurotrophic factor (GDNF). They showed that IGF-1 delays the onset of behavioural symptoms and sustains life to a greater degree than GDNF. The marked effects of IGF-1 on onset and survival were accompanied by preserved morphology of motoneurons.
Conclusion
Collectively, these findings demonstrate that IGF-1 is a central trophic growth factor, essential for muscle regeneration and hypertrophy, and motor neural maintenance and regeneration. Given the role of IGF-1 in the regeneration of nerve and muscle, it is worth further investigating the therapeutic potential of overexpression of IGF-1 in different neuromuscular diseases.
References
Van der Lely AJ, Lamberts SW, Jauch KW, et al. (2000 ). Use of human GH in elderly patients with accidental hip fracture. Eur J Endocrinol 143, 585–592.
Rinderknecht E & Humbel RE (1978). The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin.
J Biol Chem 253, 2769–2776.
Powell-Braxton L, Hollingshead P, Warburton C, et al. (1993). IGF-I is required for normal embryonic growth in mice. Genes Dev 7, 2609–2617.
Mathews LS, Hammer RE, Behringer RR, et al. (1988). Growth enhancement of transgenic mice expressing human insulin-like growth factor I.
Endocrinology 123, 282–2833.
Ye F, Mathur S, Liu M, et al. (2013). Overexpression of IGF-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol 98, 1038–1052.
Stevens-Lapsley JE, Ye F, Liu M, et al. (2010). Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab 299, E730–740.
Sheehan SM & Allen RE (1999). Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol 181, 499–506.
Barton ER (2006). Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 100, 1778–1784.
Rabinovsky ED, Gelir E, Gelir S, et al. (2003). Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J 17, 53–55.
Caroni P & Grandes P (1990). Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol 110, 1307–1317.
Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E & Delbono O (2006). Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. J Physiol 570, 283–294.
Kaspar BK, Lladó J, Sherkat N, Rothstein JD & Gage FH (2003). Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301, 839–842.
