
Physiology News Magazine
Adjunctive intracoronary imaging
Coronary heart disease is the UK’s biggest killer. The Imaging modalities discussed in this article not only play an important role in pathophysiological research, but also in the effective diagnosis and treatment of patients.
Features
Adjunctive intracoronary imaging
Coronary heart disease is the UK’s biggest killer. The Imaging modalities discussed in this article not only play an important role in pathophysiological research, but also in the effective diagnosis and treatment of patients.
Features
Kalpa De Silva Ashford
St Peter’s Hospitals NHS Foundation Trust, UK
Philippa Howlett, Fiona Hatch & Michael Mahmoudi
University of Surrey, UK
https://doi.org/10.36866/pn.95.36
Coronary angiography is an x-ray based technique whereby a catheter is passed through either the radial or the femoral artery into the coronary circulation and visualization is achieved through the injection of iodine-based contrast into the lumen under x-ray guidance. It is currently the gold standard method for imaging the epicardial coronary arteries. It remains the investigation of choice in patients suspected of having obstructive narrowings in the coronary arteries.
Such narrowings are referred to as ‘lesions’ and are characterized by the build-up of cholesterol, vascular smooth muscle cells and inflammatory cells. The efficacy of coronary angiography is limited by its two-dimensional representation of a highly dynamic three-dimensional structure. The development of adjunctive intravascular imaging techniques has enabled clinicians and scientists to gain a better anatomical understanding of any given coronary lesion, and to optimize the management of patients presenting with stable angina, unstable angina, or myocardial infarction who undergo coronary stenting as part of their treatment. The three main adjunctive intracoronary imaging modalities include greyscale intravascular ultrasound (IVUS), frequency domain optical coherence tomography (FD-OCT), and near-infrared spectroscopy (NIRS).
Intravascular ultrasound
Intravascular ultrasound (IVUS) is a catheter-based device that utilizes ultrasound frequencies of 20–40 MHz to create an image within a coronary vessel lumen (Fig. 1). The technology has been in clinical use for approximately two decades and is now the mainstream adjunctive technology in the cardiac catheterization laboratory. The technique enables precise measurements of the vessel and plaque anatomy. The addition of advanced spectral analysis, so called virtual histology IVUS (VH-IVUS), to this methodology has enabled quantification of the contents of coronary lesions into the necrotic core consisting predominantly of cholesterol-rich particles, calcium, fibrous tissue and fibro-fatty tissue (Fig. 2).
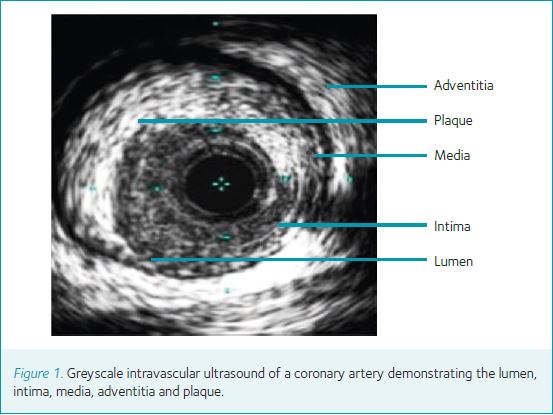


IVUS luminal area measurements may be utilized in the assessment of lesions of intermediate severity. As IVUS provides morphological assessment of coronary lesions, its role in haemodynamic assessment is limited by its inability to include lesion length and to assess lesion geometry and the amount of
myocardium supplied by the coronary artery in question. This has been borne out in recent studies demonstrating that IVUS accurately identifies non-functionally significant lesions for which coronary stenting can be safely deferred, but it cannot accurately predict haemodynamically significant lesions and therefore should not solely be used to justify treatment of a coronary lesion (Kang et al. 2011).
IVUS is being increasingly recognized as an important tool to optimize the treatment of patients undergoing coronary stenting. This is particularly the case where complex coronary artery disease is present and subsequent stenting procedures will involve complex lesions such as bifurcation lesions and lesions located within the left main coronary artery. In such populations, IVUS has been shown to reduce the rates of stent thrombosis in both bare-metal and drug-coated stent platforms, which contain anti-proliferative agents on their surface in order to reduce the rate of stent narrowing. It does so by the identification of inadequate stent expansion and/or stent apposition against the vessel wall, as well as by reducing mortality (Uren et al. 2002; Cheneau et al. 2003; Steigen et al. 2006; Doi et al. 2009; Park et al. 2009). Furthermore, VH-IVUS has been used to identify coronary lesions at risk of rupturing or eroding and leading to unstable angina or myocardial infarction (Kovanen et al. 1995).
Frequency domain optical coherence tomography (FD-OCT)
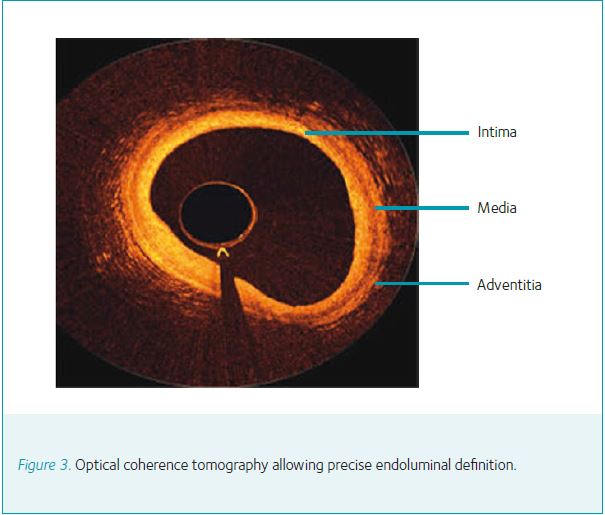
The use of VH-IVUS is being progressively surpassed by FD-OCT. This technology utilizes a near-infrared light source to image 54 mm of any blood vessel in just 2.7 s with image resolution (10–15 μm) that is 10-fold greater than IVUS (Yabushita et al. 2002) (Fig. 3). In clinical studies of patients presenting with unstable angina or myocardial infarction, FD-OCT has been demonstrated to be superior to IVUS in detecting fibrous cap disruption and erosion as well as identifying intracoronary thrombus (Kubo et al. 2007). Although FD-OCT cannot penetrate into the vessel wall as far as IVUS, it provides superior endoluminal definition and thus accurately delineates all aspects of plaque morphology including fibrous tissue, lipid accumulation and calcific deposition. In view of the improved spatial resolution, FD-OCT can be selectively used in specific scenarios.
Prior to stent implantation, FD-OCT can provide quantitative measurements of lesion length and reference vessel lumen diameter, which may guide stent selection. Moreover, when undertaken after stent deployment, FD-OCT may be used to optimize results by evaluating for under-expansion, strut
mal-apposition, and edge dissection, which are important potential substrates for stent thrombosis. FD-OCT is also playing an increasing role in the assessment and deployment of evolving stent platforms such as the bioabsorbable scaffold technology.
Near-infrared spectroscopy
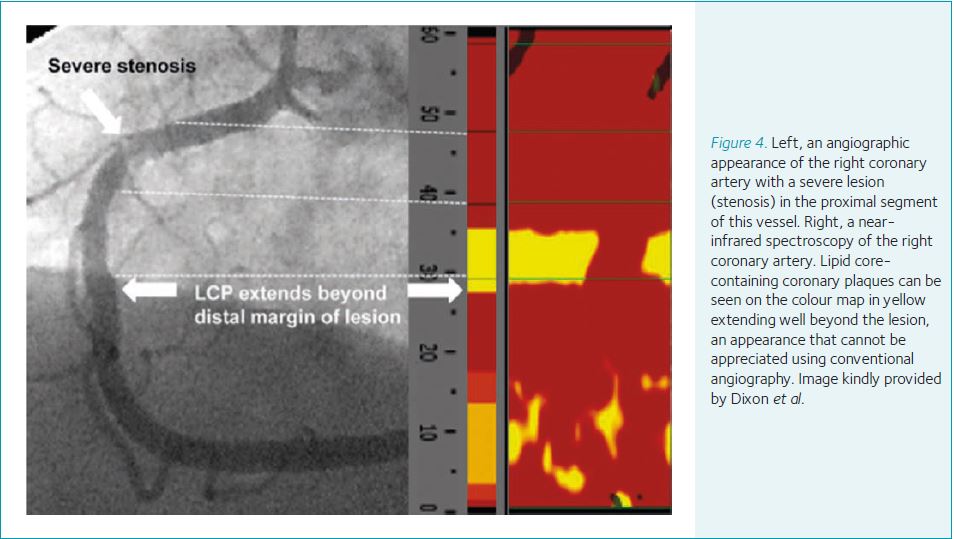
NIRS provides a colour map of the arterial wall permitting visualization and quantification of cholesterol core-containing coronary plaques (LCP), which is not seen using conventional coronary angiography alone (Fig. 4). In one study, of those lesions containing LCP, 16% were found to extend beyond conventional angiographic margins (Dixon et al. 2012). LCPs are prone to rupture and can complicate stenting procedures due to showering and obstructing the distal vasculature with lipid containing particles and lead to procedure-related myocardial infarction. In this setting, identification of LCPs using NIRS has been shown to predict adverse events. Patients with large LCPs have a 12-fold increased relative risk of peri-procedural myocardial infarction. This contrasts with a 3.5-fold relative risk using plaque characteristics assessed by standard angiography (Goldstein et al. 2011).
The clinical niche of NIRS remains to be determined. The CANARY trial (NCT01268319), a phase-2, prospective, randomized trial investigating a NIRS-guided embolic protection device to prevent peri-procedural myocardial infarction in patients with high-risk LCPs, will shed further light in the clinical applicability of NIRS.
Conclusion
IVUS, FD-OCT and NIRS have provided a paradigm shift in the invasive management of patients presenting with obstructive coronary artery disease. The clinical studies have been unanimous in demonstrating that the use of such technologies is associated with improved patient outcomes. Advances in technology are likely to ultimately provide multiple imaging modalities that can be combined on a single delivery system to allow a more comprehensive intravascular assessment and further optimize procedural outcomes. A number of studies are currently underway to determine whether characterization of any given coronary lesion using such techniques may be combined with novel, blood based biomarkers that could be used as a potential tool in identifying patients at risk of a myocardial infarction. Such patients include those with conventional risk factors for coronary artery disease such as hypertension, diabetes mellitus, hyperlipidaemia, smoking and a positive family history for coronary artery disease.
References
Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, Satler LF, Canos D, Castagna M, Weissman NJ & Waksman R (2003). Predictors of sub-acute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation 108, 43–47.
Dixon SR, Grines CL, Munir A, Madder RD, Safian RD, Hanzel GS, Pica MC & Goldstein JA (2012). Analysis of target length before coronary artery stenting using angiography and near-infra-red spectroscopy versus angiography alone. Am J Cardiol 109, 60–66.
Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, Popma JJ, Ellis SG, Grube E, Dawkins KD, Weissman NJ, Turco MA, Ormiston JA & Stone GW (2009). Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv 2, 1269–1275.
Kang SJ, Lee JY, Ahn JM, Mintz GS, Kim WJ, Park DW, Yun SC, Lee SW, Kim YH, Lee CW, Park SW & Park SJ (2011). Validation of intravascular ultrasound derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ Cardiovasc Interv 4, 65–71.
Goldstein JA, Maini B, Dixon SR, Brilakis ES, Grines CL, Rizik DG, Powers ER, Steinberg DH, Shunk KA, Weisz G, Moreno PR, Kini A, Sharma SK, Hendricks MJ, Sum ST, Madden SP, Muller JE, Stone GW & Kern MJ (2011). Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardovasc Interv 4, 429–437.
Kovanen PT, Kaartinen M & Paavonen T (1995). Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92, 1084–1088.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y & Akasaka T (2007). Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 50, 933–939.
Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, Yun SC, Lee CW, Hong MK, Lee JH & Park SW; MAIN-COMPARE Investigators (2009). Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv 2, 167–177.
Steigen TK, Maeng M, Wiseth R, Erglis A, Kumsars I, Narbute I, Gunnes P, Mannsverk J, Meyerdierks O, Rotevatn S, Niemelä M, Kervinen K, Jensen JS, Galløe A, Nikus K, Vikman S, Ravkilde J, James S, Aarøe J, Ylitalo A, Helqvist S, Sjögren I, Thayssen P, Virtanen K, Puhakka M, Airaksinen J, Lassen JF & Thuesen L; Nordic PCI Study Group (2006). Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 114, 1955–1961.
Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, Fitzgerald PJ & Yock PG; POST Registry Investigators (2002). Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J 23, 124–132.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF & Tearney GJ (2002). Characterization of human atherosclerosis by optical coherence tomography. Circulation 106, 1640–1645.
