Physiology News Magazine
The Wanderer returns: myths and realities of the cardiac vagus
For years, most textbooks have overlooked the cardiac nerve fibres in the vagus, leaving their actions almost unnoticed. But unravelling their anatomy and physiology is a key component in understanding heart dysfunction, particularly related to brain damage or disease.
Features
The Wanderer returns: myths and realities of the cardiac vagus
For years, most textbooks have overlooked the cardiac nerve fibres in the vagus, leaving their actions almost unnoticed. But unravelling their anatomy and physiology is a key component in understanding heart dysfunction, particularly related to brain damage or disease.
Features
John Coote
University of Birmingham, UK
https://doi.org/10.36866/pn.94.32
For more than a century the established idea was that the cardiac ventricles were devoid of a parasympathetic innervation. Early anatomical studies were unclear on this assertion. Here, I briefly cover the anatomical and physiological evidence showing that parasympathetic nerves, like sympathetic nerve fibres, innervate all chambers of the heart. New anatomical studies identify many cholinergic fibres distributed to both ventricles. Data show there is a complex organisation of the cardiac parasympathetic supply with a cardiotopic projection within the heart extending to the arrangement of cardiac preganglionic neurones in the medulla. Functional studies show that parasympathetic nerve excitation interacts with the sympathetic actions in the ventricle both presynaptically and postsynaptically indicating that parasympathetic terminals must extend into these chambers. Several actions of the parasympathetic nerves are described and attention is drawn to the most recent discoveries regarding an ability of vagal activity to quell life threatening ventricular arrhythmias. Evidence that this action of parasympathetic nerve fibres on the ventricles is postganglionic and due to a release of nitric oxide from non-cholinergic nerves is briefly described.
It is that which we do know which is a great hindrance to our learning that which we do not know. (Claude Bernard)
This quote reported to be from a lecture by Claude Bernard was a reference to Joseph Priestley’s discovery of the life sustaining gas in air that he referred to as dephlogisticated air. Bernard implied that Priestley missed specifically identifying oxygen because he clung to the established idea that a constituent of all combustible substances was released on burning, leaving phlogisticated air. Such a conclusion is amazing when we realize that Priestley had shown that such substances gained weight on burning in air and that the resultant compound lost weight when burned, and the released gas (oxygen) supported a flame and life. Similarly in my opinion, attachment to established opinion has resulted in many physiologists and cardiologists believing that the vagus nerve does not innervate the ventricles of the heart.
Since Willis (1664) first recognised that vagus nerve fibres supply the heart, the trademark action of this parasympathetic nerve has been a bradycardia. We now know that it does much more than just slow heart rate and mainly influence the atria. The cardiac parasympathetic nerves slow atrio-ventricular conduction, but in the ventricle they also decrease force of contraction, change action potential duration and refractory period, and are antifibrillatory.

For more than a century the cardiac vagus nerve fibres have been portrayed as limited to the atria and nodal tissue of the heart with virtually no projection to the ventricles. It seems somewhat strange that the major pumping chambers of the heart were thought to be devoid of parasympathetic nerves, whilst the atria that provide only an assist to filling of the ventricles (although nonetheless important) were densely innervated.
Frustrated by negative reviewing of many studies of the extensive actions of the vagus on the heart by our group, I decided to take a thorough look at the publications on which many early misconceptions were based. A detailed account of my examination was recently published in The Journal of Physiology (Coote, 2013). It is now clear that the vagal motor system’s influence on the heart is more complex than thought hitherto. Not only do parasympathetic nerves supply all chambers of the heart, but there is also an arrangement of the terminal projections within the heart suggesting a selective influence on different regions of the heart (cardiotopic targeting). Furthermore this topographical functional organisation of the cardiac ganglia is reflected in the grouping of preganglionic cardiac vagal neurones within the hindbrain (Fig. 1).
The cardiac vagal origins
Cardiac vagal preganglionic neurones lie in two nuclei, the dorsal motor nucleus of the vagus (DMNV) and the more ventral nucleus ambiguus (NAmb), on each side of the caudal medulla oblongata. This location was first elegantly defined by Mike Spyer and his colleagues (McAllen & Spyer, 1976). The cardiac preganglionic neurones fall into two groups, a larger group (80%) with small myelinated axons (B fibres) located in the NAmb and a smaller group with unmyelinated axons found in the DMNV and intermediate region. Each group has different discharge characteristics and their axons different conduction velocities. The preganglionic axons project in the right and left cervical vagal nerve bundles as far as the heart, where they synapse on principle postganglionic parasympathetic neurones in ganglia that affect different regions of the heart and influence different aspects of cardiac function (Fig. 1). Of course the cervical vagus nerves also contain many other parasympathetic efferents that supply the thorax and abdominal organs and it also contains postganglionic sympathetic efferent fibres. Consequently a contentious issue related to heart function has been that stimulation of the cervical vagus may also activate cardiac sympathetic fibres. However, a classical series of papers robustly demonstrated that the postganglionic sympathetic fibres coursing throughout the length of the cervical vagus nerves were not cardiac but supplied the bronchial and pulmonary blood vessels (see Coote, 2013). Cardiac sympathetic fibres from the stellate ganglion do join the vagus, but low in the neck. Stimulating these can be avoided by placing electrodes somewhere on the upper two-thirds of the cervical vagi as in the present clinical trials concerning the benefit of implanted vagal electrodes to increase cardiac vagal tone in patients with heart failure (INOVATE trial 2013).
Vagal nerve supply to cardiac chambers
A cardiac vagal influence extending into the ventricles had been viewed with scepticism because early anatomical studies appeared not to clearly demonstrate parasympathetic terminals amongst ventricular myocytes. For example Sarnoff and collaborators (1960) in an exquisite series of experiments on the action of autonomic nerves on cardiac function ignored possible actions of vagal stimulation largely due to a stated belief that parasympathetic fibres projecting so far were sparse. Perhaps as a consequence research into the actions of the cardiac vagal fibres has lagged behind that on the sympathetic influences.
Early in my career I was working with heart failure patients and I was struck by the physiological and physical benefits to these patients of regular aerobic exercise. Several mechanisms are likely to account for the cardiovascular improvements. However, an easily observable characteristic for me was a reduction in heart rate, which we now know is largely due to an increase in cardiac vagal tone. So how can an increase in vagal tone benefit a failing heart? It would not seem unreasonable to believe that in these patients the changes in vagal activity were also influencing the cardiac ventricles. This has since been implied by the physiological results of Levy and others that have clearly shown parasympathetic effects on the ventricles similar to those on the atria. Yet the area was haunted by the continued belief that the parasympathetic nerves did not project throughout the ventricles.
A more careful look at the anatomical and functional evidence shows it was not as negative as had been thought. We now understand that cardiac parasympathetic nerves do have an influence on all chambers of the heart and some actions are quite surprising.


An oft-quoted source for the absence of parasympathetic fibres in the ventricles actually concluded that fine heavily silver-impregnated fibres in autonomically decentralised young dog and cat hearts were parasympathetic (Nonidez, 1939). Support for this conclusion later came from several other groups and most substantially from studies using the thiocholine method to identify acetylcholinesterase (AChE), the enzyme responsible for hydrolysing acetylcholine (ACh). More recently renewed interest in the parasympathetic innervation of the heart prompted several studies using similar methodology. These have revealed a remarkably dense distribution of AChE stained fibres throughout all the chambers of the heart of mouse, guinea pig, rat, cat, dog, pig, sheep and human (Fig. 2; Coote, 2013). Hence it appeared reasonable to conclude that cholinergic nerves richly innervate the epicardial and endocardial surfaces of the ventricles as well as the atria. Although the evidence favours this interpretation, essential confirmation has come from recent studies using choline acetyltransferase (CHAT) immunohistochemistry. CHAT is the enzyme that catalyses the final step in the synthesis of ACh and hence is a more definitive marker of ACh nerve fibres (see Coote, 2013). These studies have successfully demonstrated a significant presence of cholinergic fibres in the ventricles (Fig. 2).
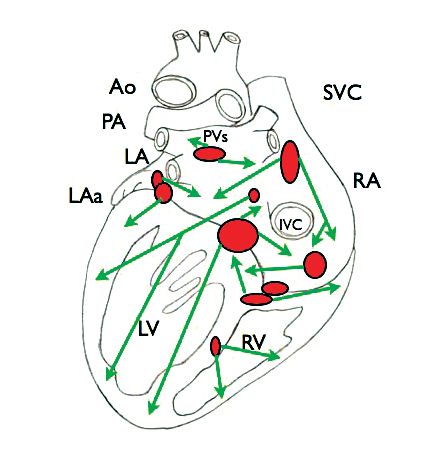
In summary, present knowledge is that the cardiac vagal preganglionic nerves pass close to the cardiopulmonary vessels and superior vena cava to synapse on ganglia located in several epicardial fat pads on the dorsum of the atria close to the site of entry of the major veins and surface of the ventricles as well as the interventricular groove and the interatrial and interventricular septa (Fig. 3; Singh et al. 1996; Coote, 2013). These ganglia are now considered the principle sites of vagal preganglionic termination and their number varies across species. However, the description is still a little confused because of the dense network of neurones that form the cardiac plexuses of an intrinsic nervous system of the heart chambers (Armour, 2008). To my mind, despite the exquisite studies of Bob Wurster and colleagues (for example Singh et al. 1996, 2009), a more comprehensive identification to reveal both pre- and postganglionic projections and location of the parasympathetic ganglia is needed.
Armed with this knowledge of a widespread cholinergic innervation of the heart it has become possible to understand why there are many studies showing a dense distribution of muscarinic receptors throughout the heart that includes both right and left ventricles. These findings are supported by convincing evidence showing that ACh is released from various postganglionic sites in the ventricles that is dependent on the strength and frequency of preganglionic vagal stimulation (Coote, 2013).
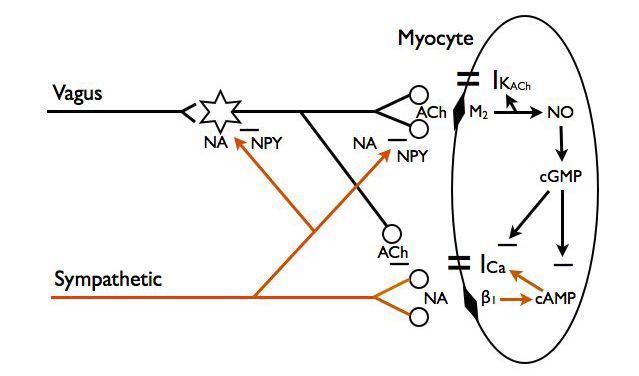
In conclusion the anatomy and pharmacological data indicate that the cardiac parasympathetic nerves not only innervate the atria, nodal and conducting tissue but also both ventricles as indicated in Fig. 4. This is further supported by functional studies.
Physiological evidence of vagal control of the cardiac ventricles
There has been considerable evidence from studies over the last 40 years that stimulation of the vagus nerve changes ventricular muscle electrophysiology and force of contraction. Despite this, even up to quite recently textbooks still depict the cardiac parasympathetic nerve supply only projecting as far as the conducting tissue. There is now substantial and robust physiological data from studies showing interaction between parasympathetic and sympathetic activity on myocardiac performance (Fig. 5).
For example the effects of vagal stimulation on reducing rate of ventricular contraction and its force, and lengthening atrial–ventricular conduction time are enhanced in the presence of sympathetic activity. Thus, in the presence of sympathetic tone, muscarinic receptor activation by parasympathetic nerve-released ACh suppresses the action of sympathetic nerves on the ventricular myocardium (Coote, 2013). Much evidence suggests this is partly a pre-junctional effect whereby ACh released from parasympathetic postganglionic terminals reduces the amount of catecholamine released from sympathetic nerves (Levy, 1977). The cardiac effect of the pre-junctional actions is enhanced by a post-junctional cholinergic receptor-dependent intracellular elevation of cGMP that then inhibits cAMP so reducing the sympathetic adrenergic-activated currents. These parasympathetic–sympathetic interactions known as accentuated antagonism have been elegantly confirmed and extended to the cellular level in Paterson’s lab (for example Dawson et al. 2008).
Additionally, there is robust evidence that parasympathetic postganglionic nerve fibres have direct effects on ventricular myocardial cells independent of sympathetic activity.
In intact animals and in an isolated innervated heart preparation vagal stimulation has been shown to lengthen the duration of ventricle monophasic action potentials and the refractory period, in the absence of sympathetic activity (Brack et al. 2007; Coote, 2013). Negative inotropic actions of the vagus after removal of sympathetic influence have also been shown in several studies. These indicate that in anaesthetised animals and humans, after beta-adrenoreceptor blockade, vagal nerve stimulation has a small but significant negative inotropic action on the ventricles (Coote, 2013). Such results have not gone unchallenged but it seems to me that a direct parasympathetic influence on ventricular muscle force would be at least as functionally important as the established negative inotropic action on atrial muscle.
Vagal modulation of ventricular rhythm
One influence of the cardiac parasympathetic activity on the ventricles that is overlooked but is well documented is a protective effect on the vulnerability for ventricular fibrillation (Coote, 2013). Part of this action may be due to accentuated antagonism of sympathetic action via parasympathetic cholinergic nerves (Fig. 5).
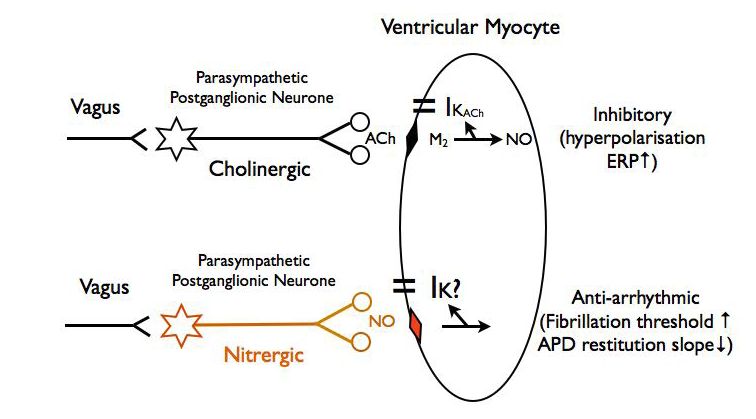
However, there is now convincing evidence supporting a direct non-cholinergic anti-arrhythmic action of vagal stimulation which has been demonstrated in the isolated innervated rabbit heart preparation in the absence of sympathetic activity (Brack et al. 2007, 2009, 2011). In confirmation of many early reports in intact animals, stimulation of the vagus nerves either prevented or increased the threshold for a stimulus to cause fibrillation. These authors also measured the vagus nerve effect on action potential duration restitution (APDR), a characteristic that reflects the probability of an electrical wave front breaking up into multiple wavelets that result in desynchronised spread of electrical activity and uncoordinated myocyte contraction. We can liken this to a sea wave breaking onto the shoreline where the slope of the beach, the wavelength and the velocity of the wave determine wave break-up. In the case of the heart, diastolic interval (slope of beach) determines the action potential duration (wavelength) and APDR measures this relationship with a higher value facilitating fibrillation. Vagus nerve stimulation decreased APDR so confirming the fibrillation threshold results. A real surprise, though, was that the raised ventricular threshold to fibrillation was unaffected by atropine but selectively blocked by a neuronal nitric oxide synthase antagonist suggesting it is mediated via NO release and is independent of ACh acting at muscarinic post-junctional receptors. In further experiments NO release was directly measured and demonstrated to be independent of ACh release. Whilst there are several possible explanations for these results, the most plausible is that the anti-arrhythmic action on the ventricle of parasympathetic terminals is exerted by a select population of postganglionic nitrergic fibres (Fig. 6). There are anatomical data from several studies showing postganglionic nitrergic nerve fibres supplying the heart chambers. For example Singh et al. (2009) calculated that 37% of human cardiac ganglion neurones contain NO synthase.
Up to quite recently we have always assumed that the cardiac parasympathetic postganglionic nerve fibres are cholinergic and are phenotypically homogeneous. However, parasympathetic fibres to the gastrointestinal tract and to the lower urinary tract and vas deferens consist of both cholinergic and nitrergic fibres (see Coote, 2013).
Therefore, the cardiac parasympathetic innervation is most unlike the common picture that is presented in textbooks. It is heterogeneous in terms of both anatomical location and projection of preganglionic and postganglionic neurones (for example Sampaio et al. 2003) and in neuronal size as well as in neurochemistry and physiology.
Interaction with intrinsic nerve plexuses
It is common to interpret neural effects on the heart as being due to the actions of the extrinsic autonomic nerves. However, the heart, like the gastrointestinal tract, also has an extensive intrinsic network of neurones that are postulated to be a ‘little brain’ (Randall et al. 1996) that is capable of powerfully modulating regional and whole heart function (Armour, 2008). The presence of the ganglion plexuses that have connections throughout all cardiac chambers adds to the complexity of the innervation of the heart. A few studies have examined the interaction of intrinsic and extrinsic nerve systems, but such participation has not so far featured strongly in interpretation of the actions of extrinsic autonomic nerves on the heart.
Conclusion
For many years most textbooks have neglected the cardiac nerve fibres in the vagus and so their many actions have gone almost unnoticed. This has to change. A full knowledge of the anatomy and physiology of the cardiac parasympathetic nerves is essential if we are to understand heart dysfunction and in particular how brain damage or disease can have serious adverse cardiac effects.
References
Brack KE, Coote JH & Ng GA (2011). Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res 91, 437–446.
Brack KE, Patel VH, Coote JH & Ng GA (2007). Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 583, 695–704.
Brack KE, Patel VH, Mantravardi R, Coote JH & Ng GA (2009). Direct evidence of nitric oxide release from neuronal nitric oxide synthase activation in the left ventricle as a result of cervical vagus nerve stimulation. J Physiol 587, 3045–3054.
Dawson TA, Li D, Woodward TD, Barber ZE, Wang L & Paterson DJ (2008). Cardiac cholinergic NO-cGMP signaling following acute myocardial infarction and nNOS gene transfer. Am J Physiol Heart Circ Physiol 295, H990–H998.
Levy MN (1977). Parasympathetic control of the heart. In Neural Regulation of the Heart, ed. Randall WC, pp. 97–129. Oxford University Press, New York.
Levy MN & Schwartz PJ (2004). Vagal Control of the Heart: Experimental Basis and Clinical Implications. Futura, New York.
McAllen RM & Spyer KM (1976). The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol 258, 187–204.
Nonidez JF (1939). Studies on the innervation of the heart. 1. Distribution of the cardiac nerves with special reference to identification of sympathetic and parasympathetic postganglionics. Am J Anat 65, 361–413.
Randall WC, Wurster RD, Randall DC & Xi-Moy SX (1996). From cardioaccelerator and inhibitory nerves to a heart brain: an evolution of concepts. In Nervous Control of the Heart, ed. Shepherd JT & Vatner SF, pp. 173–200. HAP, Amsterdam.
Sampaio KN, Mauad H, Spyer KM & Ford TW (2003). Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp Physiol 88, 315–327.
Sarnoff SJ, Brockman SK, Gilmore JP, Linden RJ & Mitchell JH (1960). Regulation of ventricular contraction: Influence of cardiac sympathetic and vagal nerve stimulation on atrial and ventricular dynamics. Circ Res 8, 1108–1122.
Singh S, Gray T & Wurster RD (2009). Nitric oxide and carbon monoxide synthesizing enzymes and soluble guanylyl cyclase within neurons of adult human cardiac ganglia. Auton Neurosci 145, 93–98.
Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna, VA, Sullivan HJ, Montoya A, Tran H, Wehrmacher WH & Wurster RD (1996). Topography of cardiac ganglia in the adult human heart. J Thorac Cardiovas Surg 112, 943–953.
Willis T (1664). Cerebre Anatome, cui accessit nervorum descriptio et usis. Flasher, London.