
Physiology News Magazine
Is the desensitization of postsynaptic receptor channels relevant?
Are ion channels more than just an open and closed case?
Features
Is the desensitization of postsynaptic receptor channels relevant?
Are ion channels more than just an open and closed case?
Features
David Papke & Claudio Grosman
University of Illinois, USA
https://doi.org/10.36866/pn.90.32
Receptor-channels involved in rapid communication between neurons have historically been thought of as simple ‘nano-machines’ that transition between closed and open states during normal neurotransmission. However, channels can also visit a third, refractory conformation, known as the ‘desensitized’ state. By quantifying receptor responses to synaptic-like stimulation, we have gained better insight into how the desensitized conformation may impact receptor responses in synapses, a result that may have implications for synaptic design.
Our brains have many trillions of chemical synapses (Pakkenberg et al. 2003). During fast synaptic transmission, presynaptic terminals emit a series of brief neurotransmitter pulses that are sensed by neurotransmitter-gated ion channels (NGICs) located in the postsynaptic cell membrane. NGICs have both an extracellular neurotransmitter-binding domain and a transmembrane pore that can alternately adopt ion-conductive (‘open’) and non-conductive (‘closed’ and ‘desensitized’) conformations. The binding of neurotransmitter favors the open conformation of the pore over the closed, and thus allows ions to pass through the channel and change the electrical potential across the membrane. The magnitude of this change in membrane voltage can decrease with each successive puff, or ‘pulse,’ of neurotransmitter despite constant stimulation of the presynaptic neuron, a phenomenon termed ‘short-term synaptic depression’ (Fig. 1). This depression can affect signal transmission because, for an action potential to be propagated to the next cell, the change in the postsynaptic membrane potential needs to surpass a threshold. Indeed, if the postsynaptic response declines enough during the course of a series, or ‘train,’ of neurotransmitter pulses, this threshold will not be overcome, and the propagation of the signal will be prevented. Short-term synaptic depression has been observed in synapses in the brain, but there is still uncertainty surrounding the causes. In fact, several distinct mechanisms have been proposed to underlie this phenomenon, including, for example, depletion of presynaptic neurotransmitter reserves and changes in neurotransmitter release dynamics over the course of the pulse train.

In our work, we have examined one such mechanism, namely, the progressive loss of receptor responsiveness over the course of a series of neurotransmitter pulses resulting from the receptors entering the ‘desensitized’ state. Like the closed conformation of the channel, the desensitized conformation is non-conductive. Unlike the closed conformation, however, the desensitized state of neurotransmitter-bound receptors is very stable, and thus, desensitized channels do not open readily. The physiological role of this refractory conformation has been a long-standing mystery in the field of ion-channel research. Out of the three superfamilies of NGICs – excitatory glutamate receptors, Cys-loop receptors and purinergic receptors, altogether comprising several dozens of channels – every known channel can desensitize upon binding neurotransmitter. Indeed, applying a sufficiently long pulse of neurotransmitter to a group of receptors will force essentially all of them into the desensitized state. However, in the body, neurotransmitter pulses are generally too short (the average duration is thought to be in the 100 ms to 1 ms range) for a large fraction of the channels to desensitize during the pulse. So, in spite of its conservation among NGICs, the desensitized state has historically been deemed physiologically irrelevant (with the AMPA-type glutamate receptors [AMPARs] being, perhaps, the best-studied exception; Trussell and Fischbach, 1989). Hence, researchers studying synaptic depression have focused on other mechanisms of controlling signal transmission in synapses, while giving desensitization relatively little consideration.
Work from a number of laboratories has shown that desensitization can affect the response of AMPARs and muscle acetylcholine receptors (AChRs) to synaptic-like stimulation in excised pieces of membrane (known as ‘outside-out patches’ – see Fig. 2). In the case of the fast-desensitizing AMPARs, some desensitization is expected to occur during brief glutamate pulses. However, it has been shown that desensitization during the pulses cannot account for the amount of depression that is seen in response to repetitive stimulation (Raman and Trussell, 1995). Recently, it has been shown that desensitization of AMPARs is critically important for brain function – a mutation that impairs desensitization is lethal in mice (Christie et al. 2010). These results beg further investigation into the role of desensitization in synaptic responses mediated by these fast-desensitizing channels.

But what about slower-desensitizing receptors? As it turns out, significant peak response depression occurs when outside-out patches containing AChRs are subjected to repetitive stimulation. By examining the effect of both lab-generated and naturally-occurring mutations, Elenes and coworkers (2006, 2009) reported that AChRs desensitize during repetitive stimulation despite the short (1 millisecond) duration of each individual pulse. In this work, it was found that mutant AChRs with prolonged deactivation time courses (that is, with longer ‘bursts’ of single-channel openings, on average) exhibit more peak-response depression over the course of a train of pulses than do wild-type receptors. This finding is consistent with the idea that receptors can desensitize during the neurotransmitter-free interpulse intervals, while neurotransmitter dissociates from the receptor’s binding sites. Of course, during this time, NGICs are ‘unaware’ of the loss of external neurotransmitter as long as their binding sites remain occupied, and so they still can desensitize. Therefore, the ability of receptors to desensitize during physiological stimulation is not limited by the duration of neurotransmitter pulses (Fig. 3).

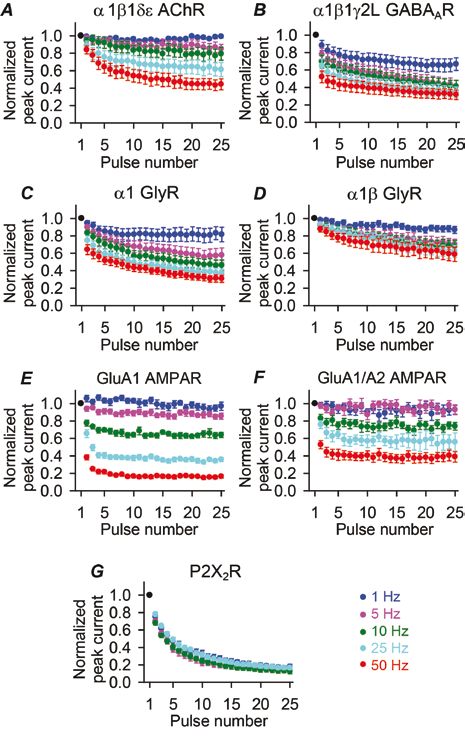
In our recent work (Papke et al. 2011), we investigated whether the conclusions from work with AMPARs and AChRs can be generalized to all NGICs. To this end, we examined all types of receptors known to be involved in fast neurotransmission – P2X2 receptors from the purinergic receptor superfamily, AMPARs from the excitatory glutamate receptor superfamily, and glycine, GABAA, and ACh receptors from the Cys-loop superfamily – using fast-perfused outside-out patches of membrane (Fig. 2B). Upon exposing these receptors to series of brief pulses at 50 Hz (a high, but physiologically relevant frequency), we observed a decline in the peak responses in every case (Fig. 1). Clearly, desensitization can affect the responses of all NGICs, not just the fast-desensitizing ones.
In order to put these results into a broader context, we need to consider another kinetic property of these receptors: recovery from desensitization. Desensitized, ligand-bound NGICs can ‘recover’ from this refractory state by returning to the closed, unliganded conformation. But recovery typically takes quite a long time, roughly on the order of hundreds of milliseconds. As the frequency of stimulation decreases, receptors have increasingly more time to recover between pulses, and as a result, there is less depression in the peak responses to stimulation (Fig. 4). Depending on the threshold for signal propagation, it is conceivable that receptors could prevent high-frequency signal transmission while allowing low frequency signals to pass through; for example, in Figure 4E, if the threshold were at half the maximal peak amplitude, then a 10 Hz signal would be propagated while a 25 Hz signal would not. Therefore, it is possible that receptor desensitization allows synapses to act as low-pass filters. Low-pass filtering behavior has been described in some synapses (Fortune and Rose, 2001), although the extent to which receptor desensitization contributes to this phenomenon remains to be determined.
Our results may superficially seem to contradict the finding that high frequency signals can pass through some synapses apparently unhindered by desensitization. However, these synapses could be structurally adapted to circumvent the limitations imposed by receptor desensitization through, for example, the use of multiple release sites with low release probabilities (note that such a system would be defined as multiple synapses by some researchers; Stevens, 2003). By having many such low-probability release sites, a synapse may succeed in repeatedly surpassing the postsynaptic threshold potential, even at high frequencies, because each individual receptor would be activated at only a fraction of the frequency of the incoming train of action potentials. Alternatively, a sufficiently low threshold for signal propagation might permit signals to pass despite a strong decline in NGIC responsiveness.
What we now know, through our work and the work of others, is that NGICs desensitize in response to brief pulses, and that, if a given set of such receptors were exposed to repetitive stimulation at physiological frequencies, then desensitization would lead to the progressive decrease of peak-current amplitudes. Along with the better-known presynaptic mechanisms, receptor desensitization could conceivably provide yet another variable for the synaptic control of signal propagation.
References
Christie LA, Russell TA, Xu J, Wood L, Shepherd GM & Contractor A (2010). AMPA receptor desensitization mutation results in severe developmental phenotypes and early postnatal lethality. Proc Natl Acad Sci USA 107, 9412–9417.
Elenes S, Ni Y, Cymes GD & Grosman C (2006). Desensitization contributes to the synaptic response of gain-of-function mutants of the muscle nicotinic receptor. J Gen Physiol 128, 615–627.
Elenes S, Decker M, Cymes GD & Grosman C (2009). Decremental response to high-frequency trains of acetylcholine pulses but unaltered fractional Ca2+ currents in a panel of ‘slow-channel syndrome’ nicotinic receptor mutants. J Gen Physiol 133, 151–169.
Fortune ES & Rose GJ (2001). Short-term synaptic plasticity as a temporal filter. Trends Neurosci 24, 381-385.
Pakkenberg B, Pelvig D, Marner L., Bundgaard MJ, Gundersen HJG, Nyengaard JR & Regeur L (2003). Aging and the human neocortex. Exp Gerontol 38, 95-99.
Papke D, Gonzalez-Gutierrez G & Grosman C (2011). Desensitization of neurotransmitter-gated ion channels during high-frequency stimulation: a comparative study of Cys-loop, AMPA and purinergic receptors. J Physiol 589, 1571–1585.
Raman IM & Trussell LO (1995). The mechanism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor desensitization after removal of glutamate. Biophys J 68, 137–146.
Stevens CF (2003). Neurotransmitter release at central synapses. Neuron 40, 381-388.
Trussell LO & Fischbach (1989). Glutamage receptor desensitization and its role in synaptic transmission. Neuron 3, 209-218.
