Physiology News Magazine
A key role of orexin (hypocretin) neurons in the fight-or-flight response
Stress increases cardiac function, ventilation and body temperature. These changes increase metabolic rate, oxygen supply and conduction velocity of nerve impulses and prepare the body for a fight-or-flight response. A part of the hypothalamus, called the ‘defense area’, has long been known to play a key role but the precise mechanisms are largely unknown. Our recent research suggests that orexin (hypocretin) neurons act as a master switch of the fight-or-flight response.
Features
A key role of orexin (hypocretin) neurons in the fight-or-flight response
Stress increases cardiac function, ventilation and body temperature. These changes increase metabolic rate, oxygen supply and conduction velocity of nerve impulses and prepare the body for a fight-or-flight response. A part of the hypothalamus, called the ‘defense area’, has long been known to play a key role but the precise mechanisms are largely unknown. Our recent research suggests that orexin (hypocretin) neurons act as a master switch of the fight-or-flight response.
Features
Tomoyuki Kuwaki
Department of Physiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan
https://doi.org/10.36866/pn.83.15

Research on neural mechanisms of state-dependent adjustments of central autonomic regulation has been sparse, despite the importance of this event from the perspective of quality of life. In addition to calm and resting states, our daily life involves many perturbations that induce active conditions such as locomotion, eating and communication. During such active periods, cardiovascular, respiratory and body temperature regulation needs to be adjusted according to the situational demands, which differ from those during resting states, by modulating or resetting homeostatic points (Kumada et al. 1990). One of the neural substrates regulating such adjustment appears to be located in the hypothalamus because stimulation of the so-called ‘defense area’ in the dorsal hypothalamus elicits behavioural ‘rage’ along with the specific autonomic responses; this was termed the ‘defense response’ (Hess, 1954).
Several neurotransmitters have been proposed to be involved in the modulation of the efferent pathways of defense responses against stressors (for review see Kuwaki & Zhang, 2010). For example, the activation of serotonin (5HT)-1A receptors in the medullary raphe reduces cardiovascular changes, and the inhibition of 5HT-3 receptors in the nucleus tractus solitarii prevents the baroreflex bradycardia inhibition during the defense response. Micro injections of adenosine into the rostral ventrolateral medulla augment the increase in blood pressure induced by electrical stimulation of the hypothalamic defense area. The pros and cons of glutamate participation in the cardiovascular component of the defense response have been a topic of debate. However, there is no report on the molecular basis of the defense response underlying the multi-faceted nature of simultaneous and coordinated changes in the cardiovascular, respiratory, sensory, thermal and behavioural parameters. Localization of orexin-containing cell bodies in the perifornical area (PFA) and dorsomedial hypothalamus(DMH), which overlap the ‘defense area’, prompted us to investigate the possible role of orexin in the defense response against stressors.
Orexins (orexin-A and orexin-B), also known as hypocretins (hypocretin 1 and hypocretin 2, respectively), are recently discovered hypothalamic neuropeptides (de Lecea et al. 1998; Sakurai et al. 1998). Although orexins were first described as hypothalamic neuropeptides that influenced appetite and consciousness, it was later found that orexins also modulate the reward process, the pain process, and autonomic regulation of the cardiovascular, respiratory and neuroendocrine systems (Kuwaki & Zhang, 2010). Orexin-containing cell bodies are restricted to the lateral hypothalamic area (LHA), PFA and DMH. On the other hand, orexin-containing nerve terminals and receptors are widely distributed in the hypothalamus, thalamus, cerebral cortex, circumventricular organs, brainstem, cerebellum and spinal cord, suggesting that the orexin neurons have widespread connections with other regions in the brain. Specifi cally, the following cardiorespiratory-related areas receive orexinergic innervation: nucleus tractus solitarii; pre-Bötzinger complex; periaqueductal grey; rostral ventrolateral medulla; intermediolateral cell column of the spinal cord; and the retrotrapezoid, hypoglossal, medullary raphe, parabrachial/Kölliker–Fuse and phrenic nuclei (Fig. 1). Moreover, orexin neurons receive inputs from the regulatory sites of the circadian clock, sleep–awake cycle, and emotional stress, such as the ventrolateral preoptic area, locus coeruleus, dorsal raphe, amygdala, bed nucleus of stria terminalis (BNST), and suprachiasmatic and tuberomammillary nuclei.
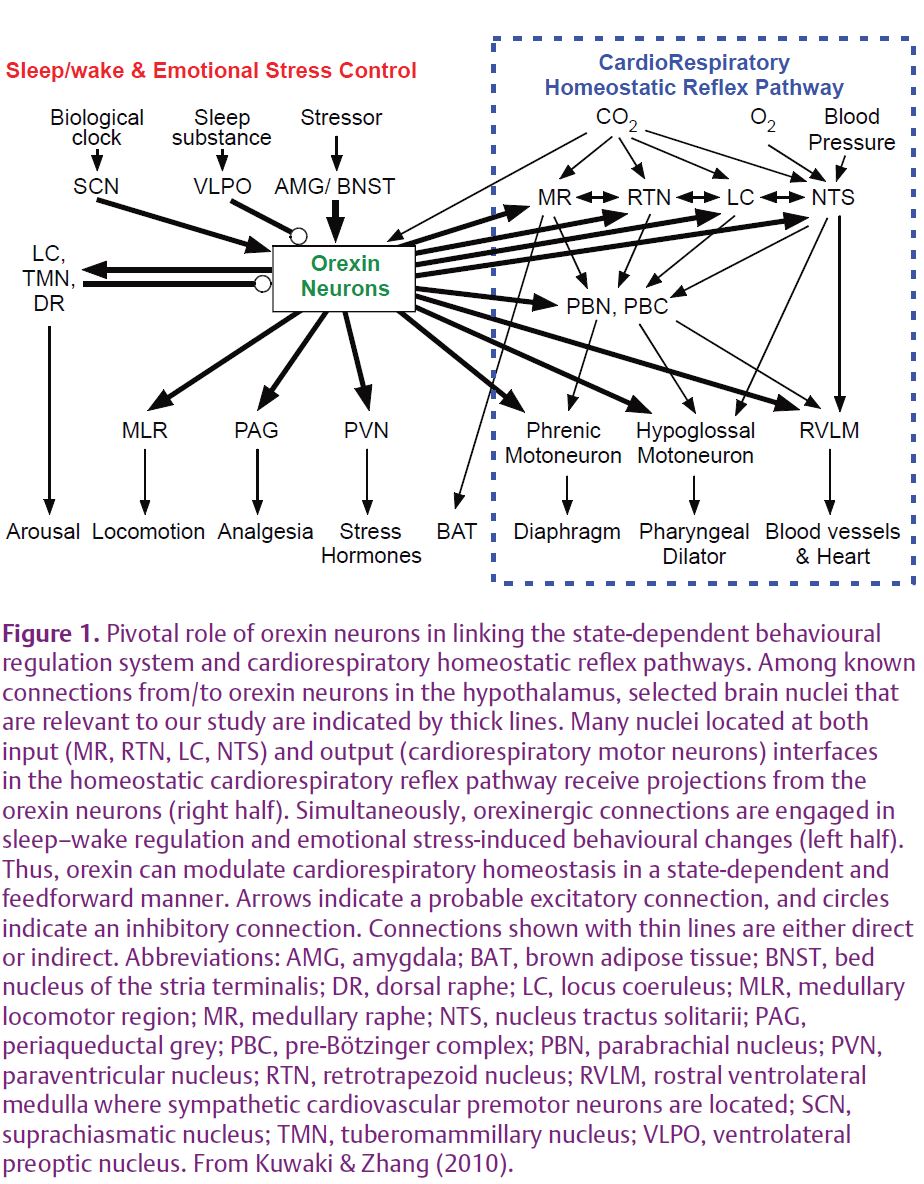
We have previously shown that orexin plays an important role in cardiorespiratory excitation during stress in orexin knockout mice (ORX-KO) and orexin neuron-ablated mice (ORX-AB) (Kuwaki & Zhang, 2010). The results of the study are summarized as follows. (1) In response to stress, both ORX-KO and ORX-AB exhibited an attenuated defense response, including increase in respiration and blood pressure and stress-induced analgesia. (2) Stimulation to the amygdala or BNST, both of which are implicated in the stress-induced autonomic responses, induced long-lasting cardiorespiratory excitation in wild-type mice but not in ORX-AB. On the basis of these results, we proposed that orexin serves as a central node between the state-dependent behavioural regulation system and the cardiorespiratory homeostatic refl ex pathways (Fig. 1).
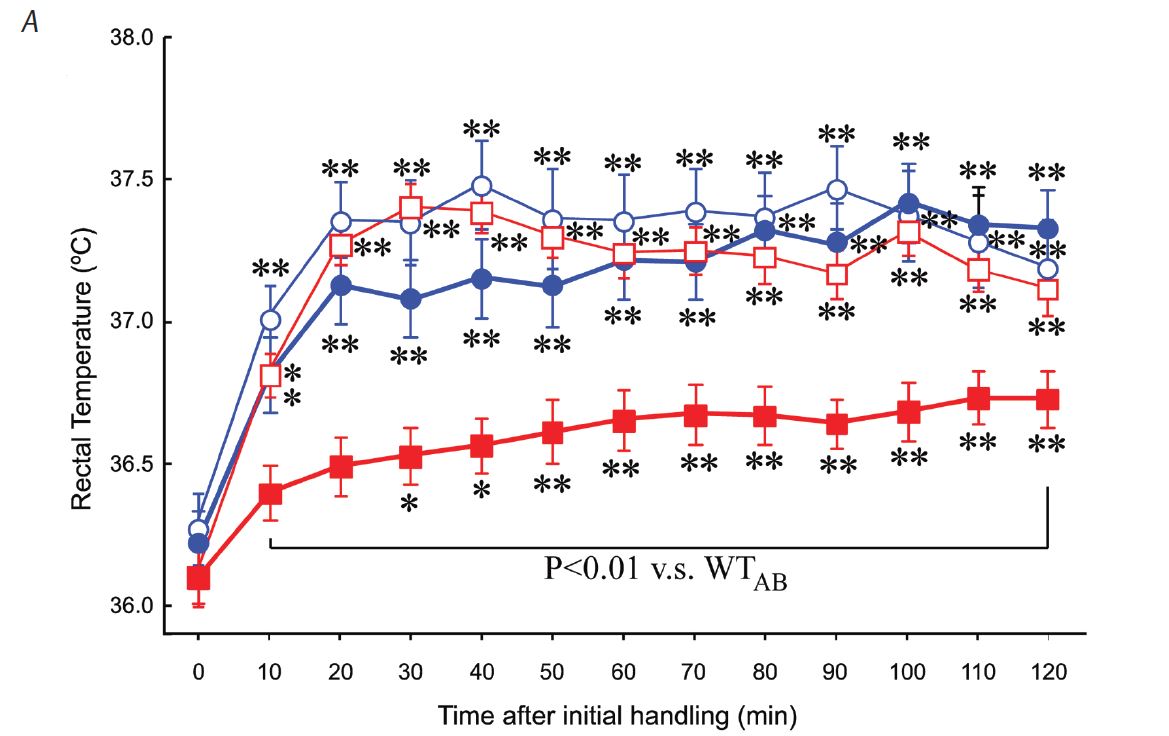
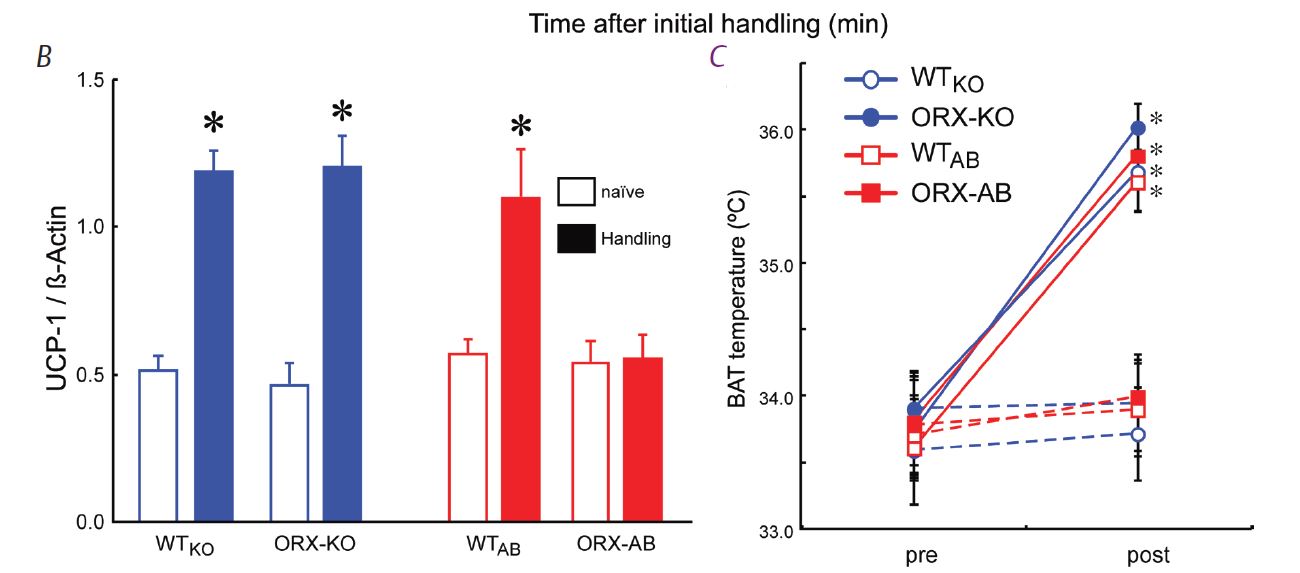
In line with the above-mentioned hypothesis, we thought that stress-induced hyperthermia would also be infl uenced by orexin. On the contrary, we found that ORX-AB but not ORX-KO mice had blunted stress-induced hyperthermia (Zhang et al. 2010) (Fig. 2A). The brown adipose tissue, which is a major thermogenic organ in rodents, did not respond to handling stress (Fig. 2B), although it did respond to a direct pharmacological stimulation (Fig. 2C). These abnormalities in ORX-AB were not observed in ORX-KO, in which orexin peptide is deficient but the neurons are preserved. Therefore, the integrity (orexin and other co-existing neurotransmitter/modulators) of the orexin neurons is indispensable for the complete expression of multiple facets of the fight-or-flight response (Fig. 3). Although the concept of co-transmitters in one neuron is well known and the related anatomical evidence has been frequently reported, the synergic/comprehensive role of the co-transmitters has been scarcely reported. From this point of view, our report is, I believe, an exceptional one.
References
de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE et al. (1998). The hypocretins: hypothalamus-specifi c peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95, 322–327.
Hess WR (1954). The Diencephalon: Autonomic and Extrapyramidal Functions. Grune & Stratton, New York.
Kumada M, Terui N & Kuwaki T (1990). Arterial baroreceptor refl ex: its central and peripheral neural mechanisms. Prog Neurobiol 35, 331–361.
Kuwaki T & Zhang W (2010). Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Resp Physiol Neurobiol 174, 43–54.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585.
Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y & Kuwaki T (2010). Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol 588, 4117–4129. http://jp.physoc.org/content/588/21/4117.long
Acknowledgements
The work from the authors was supported by the Grants-in Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports, Japan.