Physiology News Magazine
Endurance training–overtraining: a fine-tuned balance that depends on Ca2+
The razor-thin margin between beneficial effects of endurance training and the deleterious effects of overtraining is a central dilemma in endurance sports. We show here that modest changes in muscle cell Ca2+ homeostasis can cause both these effects.
Features
Endurance training–overtraining: a fine-tuned balance that depends on Ca2+
The razor-thin margin between beneficial effects of endurance training and the deleterious effects of overtraining is a central dilemma in endurance sports. We show here that modest changes in muscle cell Ca2+ homeostasis can cause both these effects.
Features
Niklas Ivarsson, Joseph D. Bruton and Håkan Westerblad
Department of Physiology and Pharmacology, Karolinska Institutet, 171 77 Stockholm, Sweden
https://doi.org/10.36866/pn.83.18

Endurance training is of fundamental importance in all types of endurance sport activities. Moreover, endurance exercise is probably the best way to counteract the large and rapidly growing health problem referred to as the metabolic syndrome: the combination of central obesity, increased blood lipids and glucose, and increased blood pressure, which often develop into type 2 diabetes and cardiovascular disease. It is then natural that there are an increasing number of studies into mechanisms by which endurance exercise mediates its benefi cial effects.
Endurance exercise results in numerous adaptations in skeletal muscle, including increased aerobic capacity and improved metabolism with increases in insulin sensitivity and fat oxidation. Studies addressing mechanisms underlying these beneficial adaptations have to a large extent been focused on cellular signalling events, such as activation of kinase cascades and transcription factors. Relatively less attention has been paid to primary effectors, i.e. factors that are directly affected by endurance exercise and subsequently activate signalling pathways and transcription factors. One tentative primary effector is increased free Ca2+ concentration in the cytosol ([Ca2+] i), because [Ca2+]i must increase to drive contractions during exercise, and in vitro experiments have shown that increased [Ca2+]i can increase mito chondrial biogenesis in adult skeletal muscle fi bres (Wright et al. 2007).
Recent studies in our laboratory were performed on cold-acclimated mice. Serendipitously, we observed adaptations in their muscles resembling both the benefi cial effects observed with endurance training and the impaired muscle function which can be the result of excessive training, i.e. overtraining (Aydin et al. 2008; Bruton et al. 2010). Both adaptations could be explained by changes in the cellular Ca2+ handling due to modifi cation of the sarcoplasmic reticulum (SR) Ca2+ release channel complex, i.e. the ryanodine receptor 1 (RyR1) protein complex (Fig. 1). The RyR1 protein complex can be modified by a wide range of stressors (Bellinger et al. 2008a), including endurance exercise and exposure to a cold environment. A critical component of the RyR1 modification is the release of the 12 kDa FK506 binding protein (FKBP12, also called calstabin 1), which results in a destabilized RyR1 channel complex and increased SR Ca2+ leak.

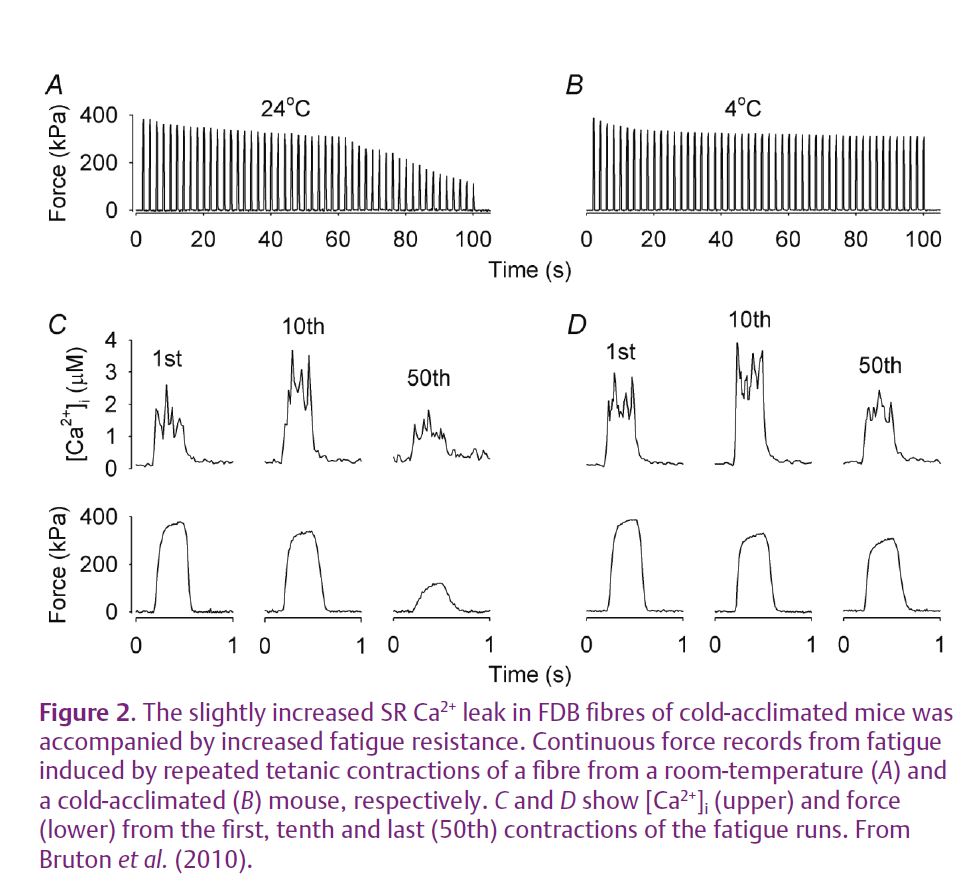
Toe muscles (flexor digitorum brevis, FDB) do not participate in the shivering response induced by exposure to a cold environment. FDB fibres of cold-acclimated mice show minor changes in the RyR1 protein complex with some increase in the RyR1 phosphorylation and depletion of FKBP12. These changes were found to be associated with increased SR Ca2+ leak and increased [Ca2+]i both at rest and during tetanic contractions. An increased mitochondrial biogenesis has previously been shown when caffeine was used to increase [Ca2+]i in muscle fibres in vitro (Wright et al. 2007). Accordingly, FDB muscles of cold-acclimated mice displayed increases in: (i) the protein expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α, a key regulator of mitochondrial biogenesis); (ii) the mRNA expression of the mitochondrial transcription factor A (Tfam, required for transcription and replication of the mitochondrial genome); (iii) the cellular mitochondrial content; (iv) the capacity for fatty acid oxidation. Furthermore, FDB fibres of cold-acclimated mice were more fatigue resistant and maintained much higher forces during fatigue induced by repeated tetanic stimulation than control fibres of mice kept at room temperature (Fig. 2). Interestingly, there was no change in fibre type (i.e. myosin heavy chain isoform) composition between FDB fibres from cold-acclimated and room-temperature mice. This is consistent with results from endurance training studies where major increases in endurance are accompanied by little or no fibre type changes, especially not between slow-twitch type I and fast-twitch type II fibres. To sum up, FDB fibres of cold-acclimated mice show increased [Ca2+]i and adaptations similar to those observed with endurance training. This occurs despite them performing no obvious endurance exercise and hence the results indicate a key causative role of increased [Ca2+]i.
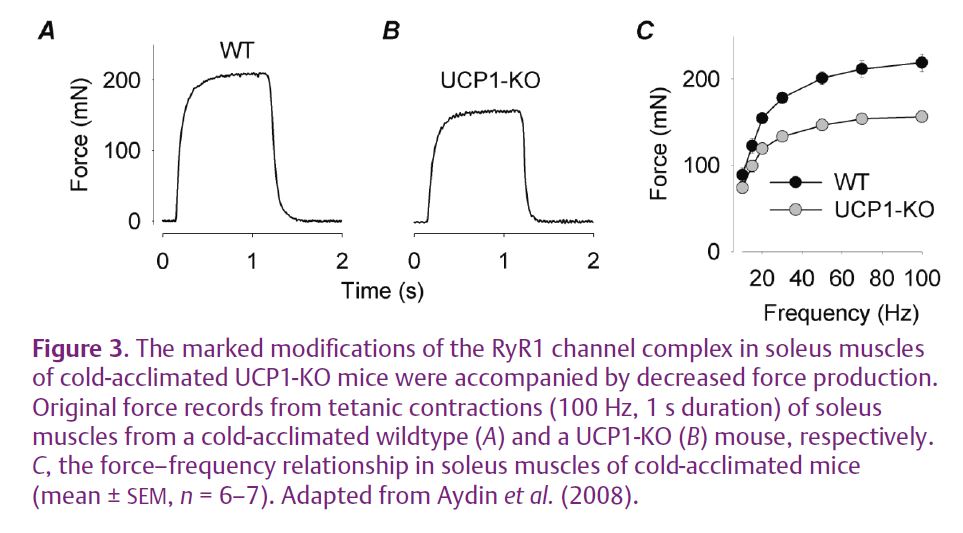
Genetically engineered mice lacking uncoupling protein-1 (UCP1-KO) cannot generate heat by increasing the metabolism in brown adipose tissue. When kept in the cold, UCP1-KO mice shiver to maintain their body temperature. Soleus muscles, which participate in the shivering response, of cold-acclimated UCP1-KO mice show major changes in the RyR1 protein complex, with hyperphosphorylation and a major FKBP12 depletion (Aydin et al. 2008). These changes were associated with decreased [Ca2+]i during contractions (see Fig. 1C) and hence also lower forces (Fig. 3). Thus, the combination of shivering and the general stress induced by exposure to a cold environment resulted in severe changes in the RyR1 protein complex and impaired contractile function. This resembles the contractile dysfunction observed with overtraining, which is a frequent problem for the elite in many endurance-type sports. This notion is supported by the fact that similar changes in the RyR1 protein complex accompanied by contractile dysfunction have been observed after a period of intense exercise (Bellinger et al. 2008b). Furthermore, similar changes may also occur in patients with disorders afflicting muscle function where muscles have to be used closer to their maximal capacity even in everyday activities.
Another interesting aspect related to exercise-induced modifications of the RyR1 channel complex and increased SR Ca2+ leak is that this would lead to accelerated energy-dependent Ca2+ reuptake into the SR and hence increased heat production. Thus, this is likely to contribute to the increase in basal metabolism and the sensation of being hot that remain for prolonged periods after many types of endurance exercise.
References
Aydin J, Shabalina IG, Place N, Reiken S, Zhang SJ, Bellinger AM, Nedergaard J, Cannon B, Marks AR, Bruton JD & Westerblad H (2008). Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J 22, 3919–3924.
Bellinger AM, Mongillo M & Marks AR (2008a). Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest 118, 445–453.
Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, Lacampagne A & Marks AR (2008b). Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A 105, 2198–2202.
Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ, Wada M, Tavi P, Nedergaard J, Katz A & Westerblad H (2010). Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol 588, 4275–4288. http://jp.physoc.org/content/588/21/4275.long
Wright DC, Geiger PC, Han DH, Jones TE & Holloszy JO (2007). Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282, 18793–18799.