Physiology News Magazine
The way we learn to walk
Learning to walk is a milestone in children’s motor skill development. Newly published research suggests that emergence of oscillatory drive in the corticospinal pathway plays an important role in developing and shaping this motor skill.
Features
The way we learn to walk
Learning to walk is a milestone in children’s motor skill development. Newly published research suggests that emergence of oscillatory drive in the corticospinal pathway plays an important role in developing and shaping this motor skill.
Features
Tue Hvass Petersen (1,2), Simon F. Farmer (2) and Jens Bo Nielsen (1)
1: Institute of Exercise & Sports Sciences, University of Copenhagen, Copenhagen, Denmark
2: Institute of Neurology, University College London, UK
https://doi.org/10.36866/pn.83.20



The world of walking
Parents regard the first few steps taken by their child as a huge milestone in their child’s motor skill development. Learning to walk independently usually occurs at around 1 year of age with some degree of variance. However, children can be taught to walk at a slightly earlier age by means of motor practice. One of the most striking examples comes from an anthropological study by Super (1976). He studied children from a particular tribe in Kenya and found that they learned to walk slightly earlier than American children and this was likely to be due to the fact that these Kenyan mothers spent a remarkable amount of time teaching their children how to walk by means of jumping-like movements and supported walking practice. Furthermore, he reported that these children seemed to spend less of their waking time lying down and hence learned to crawl later than their American counterparts (this runs contrary to the notion of a child needing to learn to crawl before it learns to walk). The final motor outcome of teaching children to walk independently a few weeks earlier than normal is unclear. However, understanding the maturation and plasticity processes of the underlying neural networks responsible for generating the characteristic pattern of gait will prove helpful in developing rehabilitation strategies for adults and children learning to walk following damage of the central nervous system.
Infant stepping – the first few steps
If you happen to have an infant in your near vicinity while reading this, now would be the right time to lift him or her and perform one of the regular exercises performed by African mothers – simply place the legs on a surface and perhaps jump the infant gently up and down. You would be likely to experience the legs performing stepping-like movements. These movements occur if the infant is younger than 2–3 month of age, for reasons we will touch upon later. Scientists have wondered which part of the nervous system generates these movements since cerebral projections and hence the brain’s control of the leg muscles has not fully developed at this stage.
Work done by Hans Forssberg (1985) showed that when very young infants (0–18 months of age) perform stepping-like movements, they place the foot on its forepart straight under the body. This is unlike adult human walking which is characterised by the foot being placed in front of the body with a clear initial heel strike. In adults, the soleus and gastrocnemius muscles act on the ankle joint in the later parts of the stance phase to propel the body forward and the tibialis anterior (TA) muscle is responsible for lifting the forefoot during the swing phase of gait to prevent it from striking the ground. However, when Forssberg investigated the individual muscle activation patterns of the infants he found that the electromyographic (EMG) activity displayed less modulation than in adults through the gait cycle and that the different muscles were often activated in parallel rather than in a sequence. He argued that these premature stepping movements are generated by pattern generators located in the spinal cord. Such pattern generators can produce alternating muscle activity in flexor and extensor muscles in the same leg and even coordinated activity between flexor and extensor muscles of both legs. In other human studies, indirect evidence of spinal pattern generators is provided by studies such as that of Yang et al. (2004). In this experiment infants stepped on a treadmill during which reciprocal organisation of muscle activity between the two legs was demonstrated and this activation pattern was modulated by the speed of the treadmill, suggesting the presence of a spinal central pattern generator that is modulated by proprioceptive input. Whilst in babies there are important biomechanical constraints on the actual output of spinal pattern generators during reflex stepping, it is acknowledged that there exists significant spinal organisation for walking which is increasingly modulated by cortical circuitry as the child grows and develops. The process of this ‘corticalization’ of lower limb control and the role of the pattern of descending neural drive is only just beginning to be understood.
Maturation of gait – the role of motor network oscillations
Although children are able to walk independently after the first few years of life, the adult pattern of gait may not be fully established until 12 to 15 years of age. The likely reason for this is that different neural pathways mature at different times during development. One important part of this is the development of the corticospinal pathway. White matter density changes in this tract continue until the age of 17 and the neurophysiological properties follow the same trajectory of development i.e. there is maturation of lower extremity muscle response to transcranial magnetic brain stimulation (TMS) (Muller et al. 1991; Eluvathingal et al. 2007). The most striking evidence of the importance of the corticospinal tract is the fact that patients with damage to the corticospinal tract either within the brain or the spinal cord display varying degrees of gait impairment depending on the extent and location of the lesion. The severity of gait impairment seen in spinal cord damage in adults suggests that once connections from brain to the spinal cord have developed the functional relevance of a central pattern generator is reduced because the system now relies on complex interactions between cortical and spinal activity.
The TA muscle motoneurones receive prominent drive from the corticospinal pathway and damage to this system, for example, in stroke, is clinically evident in the upper motoneurone ‘dropped foot syndrome’ where the forefoot is lifted poorly during the swing phase. Activity in TA and the role of corticospinal drive is easy to study both through examination of the TMS evoked response during static contraction and walking and through study of the common drive to TA motoneurones. Individual motoneurones fire at frequencies from 8 to 13 Hz; however, their activity is commonly modulated by the cortical oscillations at a frequency of around 20 Hz (beta frequencies). When looking at signals from individual pools of motoneurones it is possible to calculate how the signals are correlated in the frequency domain. Coherence analysis provides an estimate of the magnitude of common input to the different pools of motoneurones and during static muscle contraction coherence is present in the ~20 Hz beta band. Beta rhythms are coherent between the EEG/MEG and the EMG, and damage to the corticospinal tract (see, for example, Barthelemy et al. 2010) reduces or abolishes the ~20 Hz coherence between pairs of motoneurones and between the EMGs of synergistic muscles indicating that beta band coherence within and between the EMGs of muscles reflects the oscillatory modulation of corticospinal drive to spinal motoneurones.
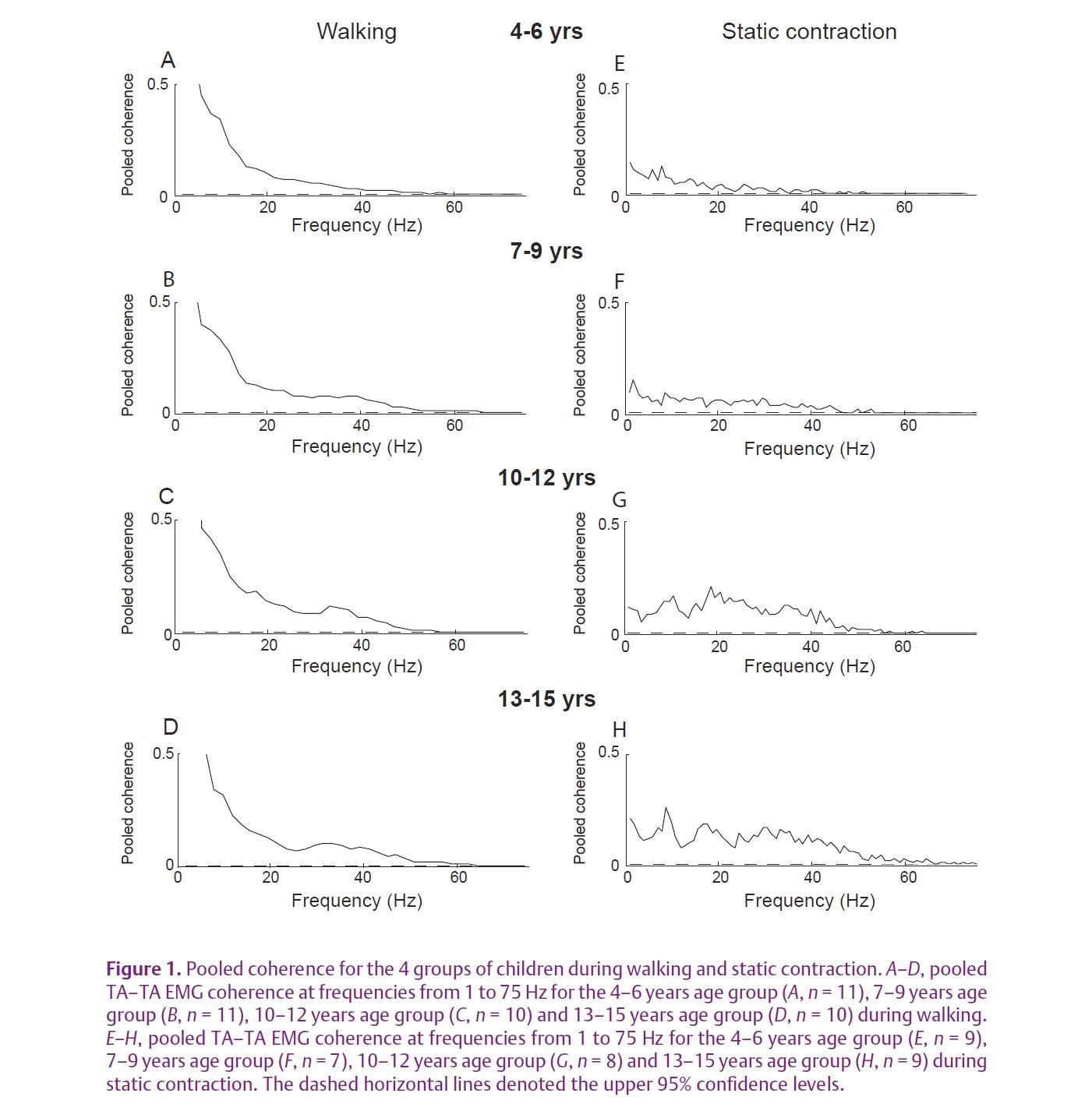
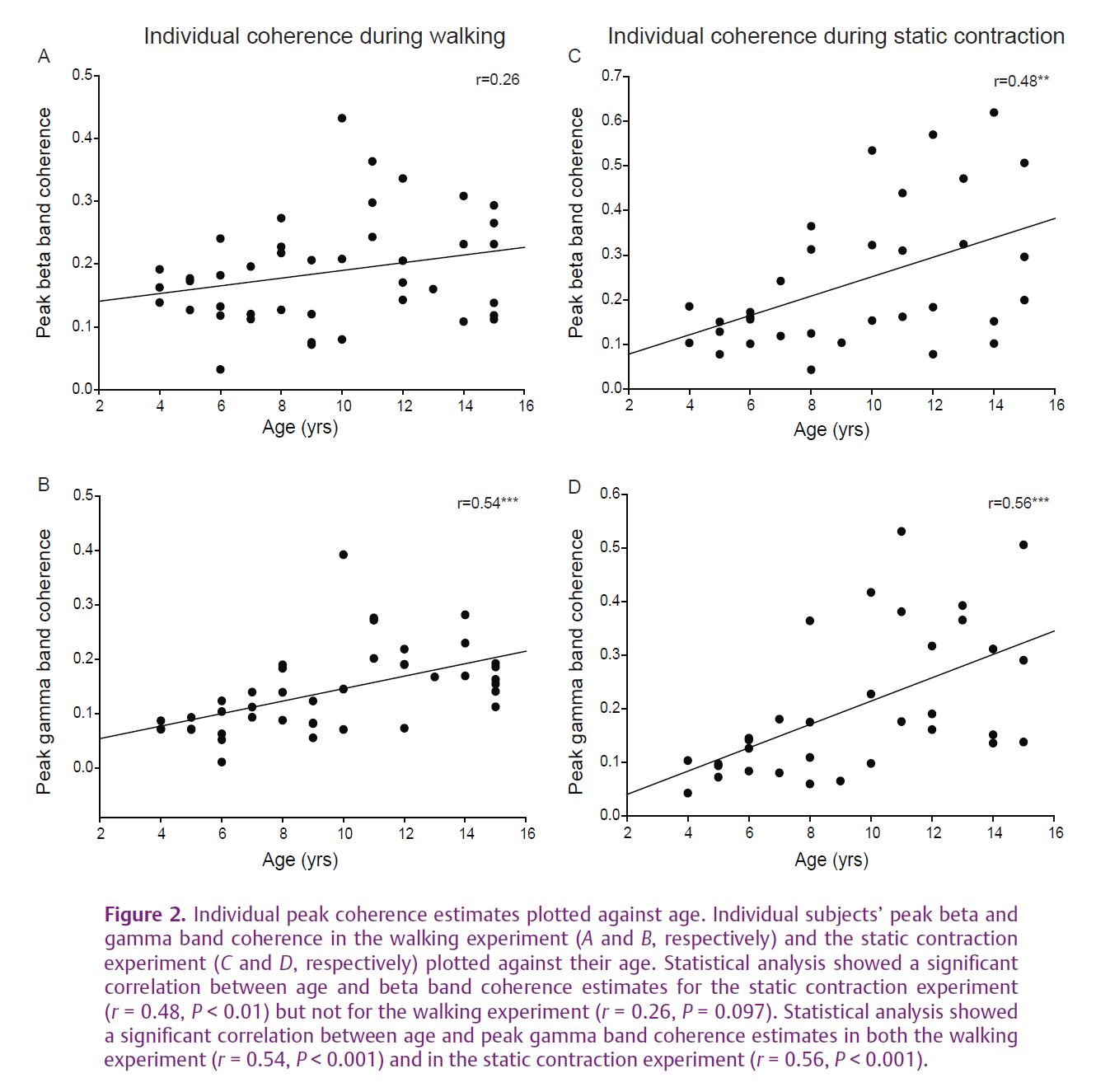
We have recently asked whether the ‘corticalization’ of muscle control during development can be reflected as an increase in the strength of the ~20 Hz common drive to motoneurones. A developmental increase was recently demonstrated for EMG–EMG and EEG–EMG coherence in upper limb muscles (Farmer et al. 2007; James et al. 2008). In a new set of experiments (Petersen et al. 2010), we investigated the development of oscillations in the corticospinal pathway during human treadmill walking in forty-two children of different ages. Firstly we showed an age-dependent increase in the ~20 Hz oscillatory input to different pools of TA motoneurones during steady muscle contraction (a result analogous to that described for the upper limb) (see Fig. 1E–H). More importantly we found strong age effects for activation of TA muscle during treadmill walking. These effects were detected for oscillatory drive at higher frequencies – the 35-40 Hz gamma band which ina number of studies has been associated with important motor cortex network functions and which can also modulate motoneurone firing. In contrast to static muscle contraction in which beta band coherence dominates, during walking the gamma band coherence dominates (Fig. 1A–D). The gamma band coherence increase was most obvious when comparing the youngest group of children (aged 4–6) with children aged between 7 and 15 years. Interestingly, individual values of peak coherence in the beta and gamma bands display a linear correlation with age (see Fig. 2). In addition we found a relationship between the strength of gamma band coherence and a measure of the efficacy of TA activation during walking, suggesting that oscillations in this frequency range are helpful in stabilizing the ankle during the swing phase of gait. This behavioural measure matures with increasing age. We argued that this age-related increase in oscillations is related to functional maturation of the corticospinal network involved in controlling the muscles during gait. The functional role of development of beta and gamma oscillations still requires investigation, and direct recordings of EEG–EMG coherence during the development of gait remain to be performed.
Gamma band coherent oscillations are important in processes requiring attention and prediction. Beta band coherence oscillations are important for maintenance of a motor state. The findings that coherent oscillatory drive to TA muscle in beta and gamma ranges changes significantly across childhood leads us to speculate that these frequencies play a crucial role in motor control. Their emergence correlates with the onset of the adult pattern of walking in which the interaction between spinal and cortical circuits becomes more fixed. Seeing how this oscillatory drive changes more precisely at the crucial transition points of motor development and their relation to learning and plasticity will be an interesting topic for further research.
References
Barthelemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sorensen F & Nielsen JB (2010). Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol 104, 1167–1176.
Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM & Ewing-Cobbs L (2007). Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17, 2760–2768.
Farmer SF, Gibbs J, Halliday DM, Harrison LM, James LM, Mayston MJ & Stephens JA (2007). Changes in EMG coherence between long and short thumb abductor muscles during human development. J Physiol 579, 389–402.
Forssberg H (1985). Ontogeny of human locomotor control. I. Infant stepping supported locomotion and transition to independent locomotion. Exp Brain Res 57, 480–493.
James LM, Halliday DM, Stephens JA & Farmer SF (2008). On the development of human corticospinal oscillations: age-related changes in EEG–EMG coherence and cumulant. Eur J Neurosci 27, 3369–3379.
Muller K, Homberg V & Lenard HG (1991). Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr Clin Neurophysiol 81, 63–70.
Petersen TH, Kliim-Due M, Farmer SF & Nielsen JB (2010). Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol 588, 4387–4400. http://jp.physoc.org/content/588/22/4387.long
Super CE (1976). Environmental effects on motor development: The case of African infant precocity. Dev Med Child Neurol 18, 561–567.
Yang JF, LamT, Pang MY, Lamont E, Musselman K & Seinen E (2004). Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol 82, 662–674.