Physiology News Magazine
A novel way to test human motoneurone behaviour during muscle fatigue
Features
A novel way to test human motoneurone behaviour during muscle fatigue
Features
Chris J. McNeil, Peter G. Martin, Simon C. Gandevia and Janet L. Taylor
Neuroscience Research Australia and University of New South Wales, Randwick, NSW, Australia
https://doi.org/10.36866/pn.82.29
Descending voluntary drive during a sustained contraction makes it difficult to test the true effect of fatigue on the excitability of spinal motoneurones. Transient, artificially induced interruption to voluntary drive enables assessment of motoneurone behaviour during muscle fatigue. This technique reveals a profound reduction in motoneurone responsiveness not seen during intact voluntary drive.


Fatigue is one of the most common symptoms reported to general practitioners. Although it may be difficult to separate general malaise from muscle fatigue in some circumstances, muscle fatigue is an unavoidable consequence of life. This is true across the full spectrum of activity, from simple daily tasks such as standing or climbing stairs to voluntary exercise or training. A commonly accepted definition of muscle fatigue is ‘any exercise-induced reduction in the ability to exert muscle force or power regardless of whether or not the task can be sustained’ (Bigland-Ritchie & Woods, 1984). It is well known that muscle fatigue has both central and peripheral components which occur in the central nervous system and working muscle, respectively. Changes within the muscle usually have the greater contribution to muscle fatigue and as a consequence much more research has been devoted to peripheral rather than central fatigue. However, it has been estimated that as much as 25% of force loss can be attributed to nervous system changes during a sustained 2 min maximal voluntary contraction and this proportion is even greater for weaker sustained contractions (Taylor & Gandevia, 2008). Despite this significant contribution, the proportion of central fatigue which resides in the motor cortex versus spinal cord remains unclear.
One method to test for fatigue-related changes at the level of the spinal cord in humans is to stimulate the corticospinal tract non-invasively at the cervicomedullary junction. This form of stimulation is usually accomplished by fixing electrodes behind the ears to the mastoid processes and passing an electrical current pulse between the electrodes. The stimulus produces a short-latency cervicomedullary motor-evoked potential (CMEP) in the electrical signal recorded from the target muscle. As the stimulus is delivered below the level of the motor cortex, a CMEP can be used to assess motoneurone excitability without the need to consider cortical changes induced by fatigue.
Using this method of stimulation, we have demonstrated that CMEP size decreases during the last 30 s of a sustained 2 min maximal voluntary contraction due to a reduced excitability of the motoneurone pool (Butler et al. 2003). What mechanisms are responsible for this fatigue-related reduction in excitability of the motoneurones during a sustained maximal effort? We proposed two candidates: (1) a change to intrinsic motoneuronal properties caused by their repetitive discharge during the sustained maximal effort; and (2) disfacilitation of the motoneurones caused by a fatigue-related reduction of excitatory input from muscle afferents. However, the question of mechanism is difficult to address because motoneurone responsiveness is strongly affected by the presence and magnitude of descending voluntary drive from the brain and it is uncertain if the level of this drive that reaches the motoneurones increases, decreases or has no net change with fatigue. Hence, we wanted to assess motoneurone excitability without, or with minimal influence from, the confounding factor of an unknown level of descending voluntary drive. We recently used a paired-stimulus protocol in an attempt to accomplish this (McNeil et al. 2009).
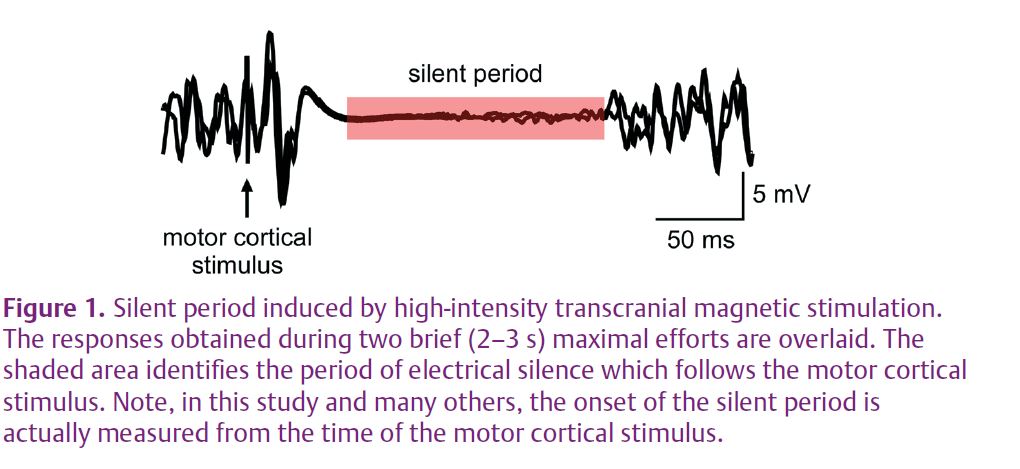
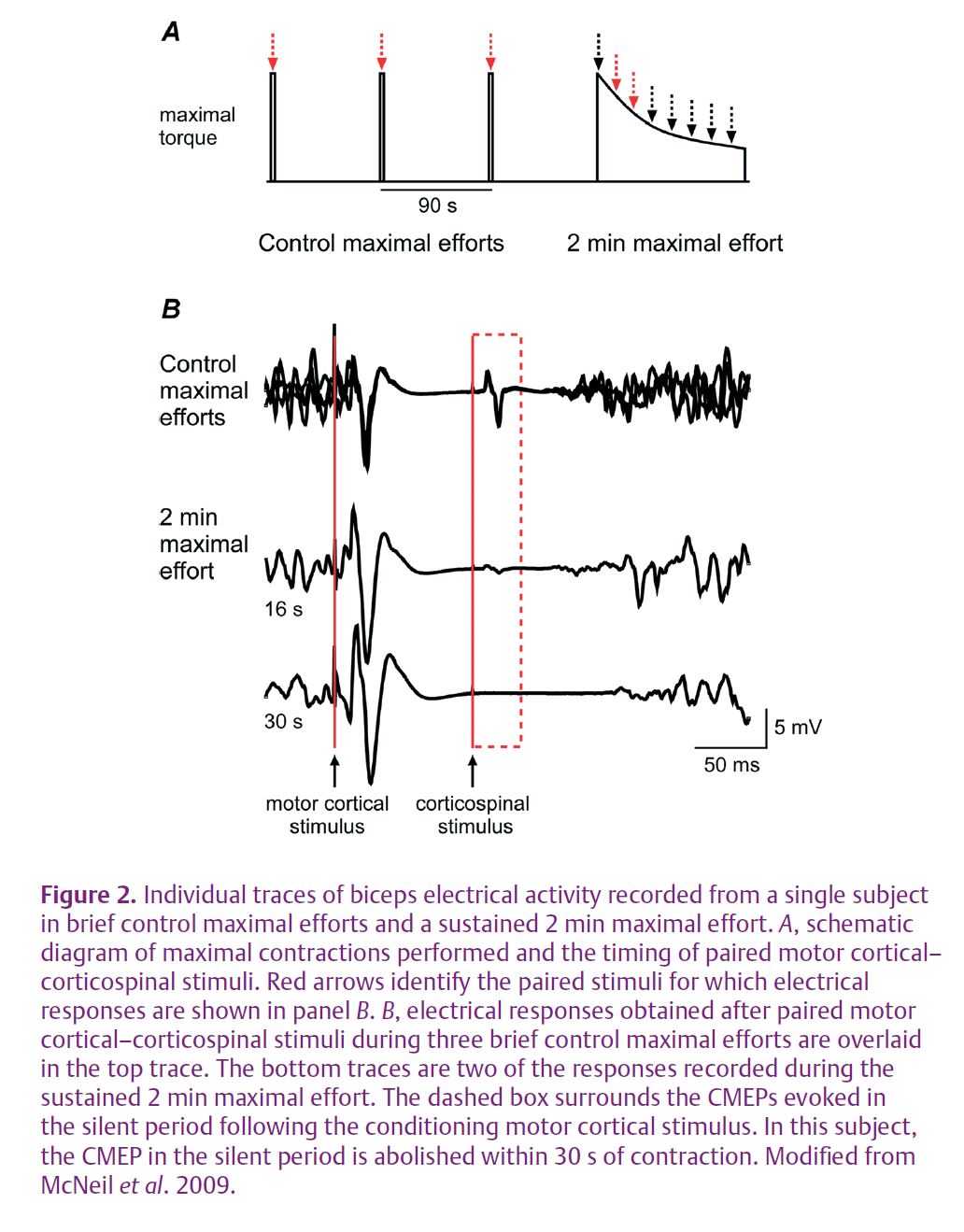
Transcranial magnetic stimulation is a non-invasive means to activate neurones within the motor cortex. A stimulating coil that produces a magnetic field is held against the appropriate region of the scalp. When a strong stimulus is delivered during a voluntary contraction, the discharge of the coil affects cortical output cells and transiently interrupts descending voluntary drive. This stops output from the motoneurones and causes a period of silence in the electrical activity recorded at the target muscle (Fig. 1). If a corticospinal stimulus were delivered in this silent period, motoneuronal properties could be tested during the development of fatigue without the complication of unknown levels of descending drive. Thus, we paired a corticospinal stimulus with a strong transcranial magnetic stimulus delivered 100 ms earlier. This pair of stimuli was given at regular intervals throughout a sustained 2 min maximal effort to assess the effect of fatigue on motoneurone excitability during the silent period (Fig. 2A). Raw traces showing CMEPs during the silent period are displayed for a single subject in Fig. 2B.
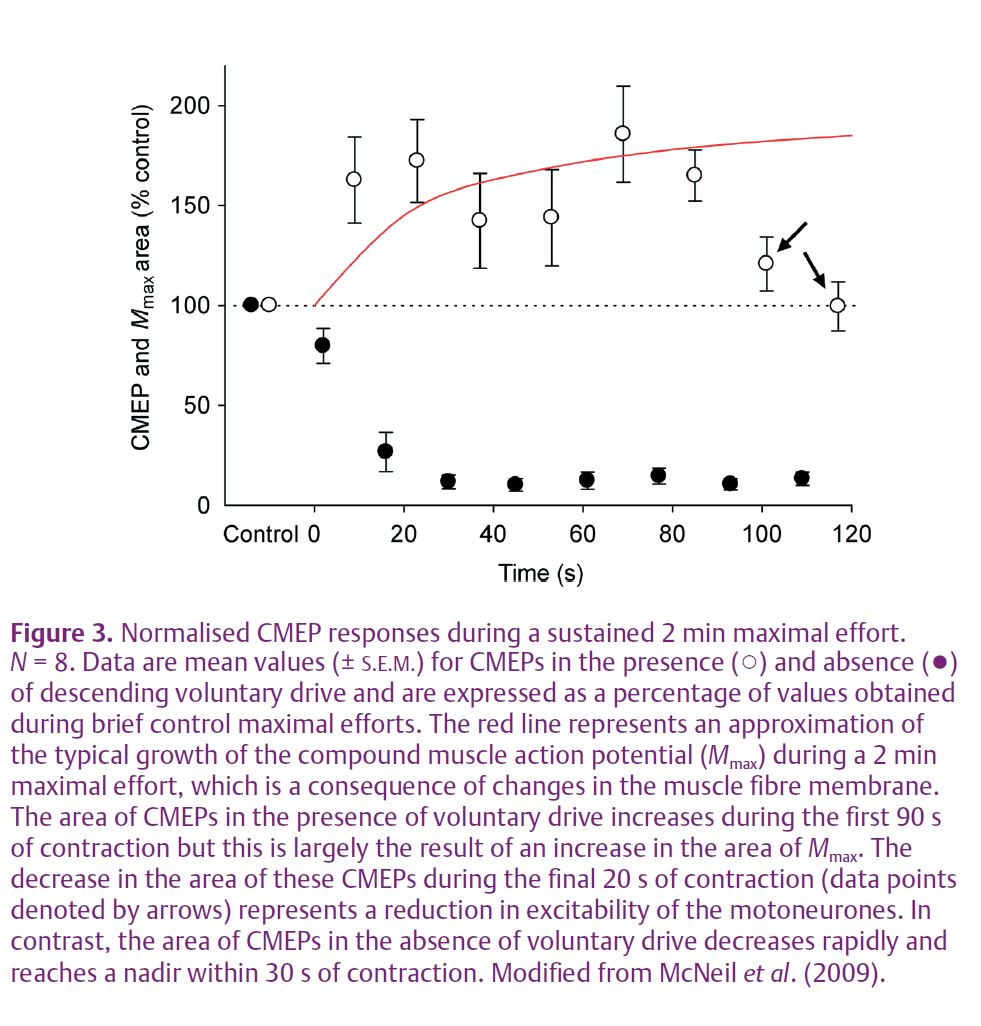
In the absence of descending voluntary drive from the brain, there is a rapid and profound decrease of motoneurone excitability during a sustained maximal effort (Fig. 3, filled symbols). This result is in stark contrast to the previous and current finding that motoneurone excitability (normalised to the increase of the peripheral potential; Fig. 3, red line) shows no change for the first 90 s of maximal effort when assessed in the presence of descending voluntary drive (Fig. 3, open symbols). A comparison of fatigue-related changes to CMEPs recorded during the silent period to CMEPs recorded during ongoing voluntary drive (Fig. 3, filled and open symbols) demonstrates the potency of descending voluntary input to the motoneurones. Namely, the facilitatory effects of this input delay any manifestation of reduced motoneurone responsiveness until 90 s of maximal effort have elapsed. Further, the decrease in responsiveness seen after 90 s is still markedly attenuated compared to the reduced excitability seen in the silent period less than 20 s into a sustained maximal contraction.
Despite the ability to provide novel insight into the effects of fatigue on motoneurone excitability, the combined use of transcranial magnetic stimulation and corticospinal tract stimulation is necessarily non-invasive and hence it remains difficult to identify the mechanisms responsible for the marked fatigue-related reduction of motoneuronal excitability. Recall that our earlier experiment with intact descending voluntary drive (Butler et al. 2003) led to two proposed mechanisms, a change to intrinsic motoneuronal properties and a reduction of excitatory input from muscle afferents on to the motoneurones. It is unacceptably invasive to obtain a direct recording of intrinsic motoneurone properties in a human, but intracellular recordings from animals show that motoneurones adapt to a constant-current input and discharge at a reduced rate due to altered conductances and a lower input–output gain of the motoneurone pool (see Powers & Binder, 2001 for review). Such processes, and no doubt others, are at the heart of the generic term changes to intrinsic properties of motoneurones. The limits to probing these changes in humans prompted us to examine the role of the reduced excitatory input from muscle afferents. We tweaked our previous protocol (seen in Fig. 2A) to include intermittent tendon vibration to increase muscle afferent input to the motoneurones during the silent period. Tendon vibration strongly excites muscle spindle afferents which should provide excitatory input to the motoneurones innervating the muscle in which the afferents are located. Preliminary findings indicate that vibration during a sustained maximal contraction does not change CMEP size. A possible interpretation is that reduced muscle spindle input has only a limited contribution to the profound fatigue-related decrease of motoneurone excitability.
In summary, a test of motoneurone responsiveness after interruption of descending voluntary drive shows that the facilitatory effect of this input to the motoneurones masks a profound and rapid decrease of motoneurone excitability during a sustained maximal effort. We believe that a stimulus to the corticospinal tract paired with a preceding strong stimulus to the motor cortex is a promising technique to explore spinal contributions to central fatigue in humans.
References
Bigland-Ritchie B & Woods JJ (1984). Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7, 691–699.
Butler JE, Taylor JL & Gandevia SC (2003). Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23, 10224–10230.
McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2009). The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587, 5601–5612. http://jp.physoc.org/content/587/23/5601.long
Powers RK & Binder MD (2001). Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143, 137–263.
Taylor JL & Gandevia SC (2008). A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104, 542–550.