Physiology News Magazine
Phantom hands: a window into how we perceive limb position and movement
The senses of limb position and movement are critical to the brain’s ability to control movement of the body. These senses are derived from multiple signals and previously it was thought that all these signals came from the muscles, skin and joint. Recent work using ‘phantom’ limbs in healthy subjects has shown that signals originating in the brain itself also contribute to the senses of limb position and movement.
Features
Phantom hands: a window into how we perceive limb position and movement
The senses of limb position and movement are critical to the brain’s ability to control movement of the body. These senses are derived from multiple signals and previously it was thought that all these signals came from the muscles, skin and joint. Recent work using ‘phantom’ limbs in healthy subjects has shown that signals originating in the brain itself also contribute to the senses of limb position and movement.
Features
Lee D. Walsh, Simon C. Gandevia and Janet L. Taylor
Neuroscience Research Australia and the University of New South Wales, Sydney, Australia
https://doi.org/10.36866/pn.81.34
A healthy person will always know the position and posture of their body in space. Even with our eyes closed we know the position and size of our arms, legs, fingers, head, etc. This is possible because the brain maintains a ‘body schema’, a representation of our body and its position in space. This body schema cannot simply be a fixed ‘map’ of our body as our bodies are constantly changing position and size. Some of our body’s parameters change slowly. The length of our limbs, the size of our muscles or circumference of our waist changes as we grow, exercise and eat. However, even the increasing length of an adolescent’s legs during a growth spurt is very slow compared to the changes that our body schema has to follow when we move our limbs because limb movements cover large distances in fractions of a second. The relatively slow changes to limb length and size could easily be updated in the body schema over time by vision and other senses and, because such changes are small over long periods of time, it is not so critical for the body schema to track these precisely. However, the large and fast changes in joint angle that are associated with every voluntary movement must be tracked continuously and accurately so that we know at all times where our body and limbs are in space. This ability is critical to the control of body movements. To perform the simple task of picking up a glass of water we must know the position of our fingers, hand and arm, in addition to the location of the glass. It can be said that we know all these things because we can see our fingers, hand and arm as it picks up the glass. That is true, but if you know where the stationary glass is, you can pick it up with your eyes closed. Furthermore, you can still do this if there are unknown obstacles and perturbations between your hand and the glass. This means that your brain can track the position and movements of your arm, hand and fingers without the aid of vision. How is this done?
One way the brain can rapidly update the body schema regarding movements is with information coming from the periphery. Classically the peripheral receptors in the skin form the basis of our sense of touch. In addition to this role, some skin receptors also signal how much the skin stretches around a joint as it moves and thus provide useable information about joint movement and position (e.g. Collins et al. 2005). Muscle spindle receptors can signal the length of muscles as well as their rate of change of length. Muscle spindles are sensitive to muscle vibration and classic experiments by Goodwin et al. (1972) showed that vibration of limb muscles induces illusory movements of the limbs. From the length of its surrounding muscles, the angle of the joint can be determined and from the rate of change of length of the muscles the velocity of joint movements can be determined. Interpretation of any muscle spindle signal from a contracting muscle is not simple because the size of the signal is modified in complex ways by the fusimotor system. The signal also diminishes if the muscle shortens too rapidly. Receptors located in the joint capsule can also contribute information about joint movement. All these signals travel through large-diameter nerves to the brain, which means that the information gets there very fast, as is required to keep the brain’s body schema updated with ongoing movements. These signals from the muscles, skin and joints provide critical information about joint movement, and losing this information results in serious disability (Cole, 1991). However, input from the peripheral receptors is not the only way the body schema is updated during movement.

Amputees often continue to perceive a limb or body part that has been removed. These sensations are referred to as ‘phantoms’ and can be explained by the body schema persisting in the brain, despite the limb having been amputated and no longer providing meaningful sensory information about itself. Another interesting thing about phantom limbs is that they can be ‘moved’. Often this is described as a phantom which itself is static but which moves along with the remaining part of the limb or a phantom that moves when partially amputated muscles, that were once involved in moving the missing limb, are activated (e.g. Reilly et al. 2006). However, some amputees are able to make movements of joints within a phantom that has none of its relevant musculature remaining. For example, an amputee whose arm is amputated at the shoulder may make phantom finger movements.
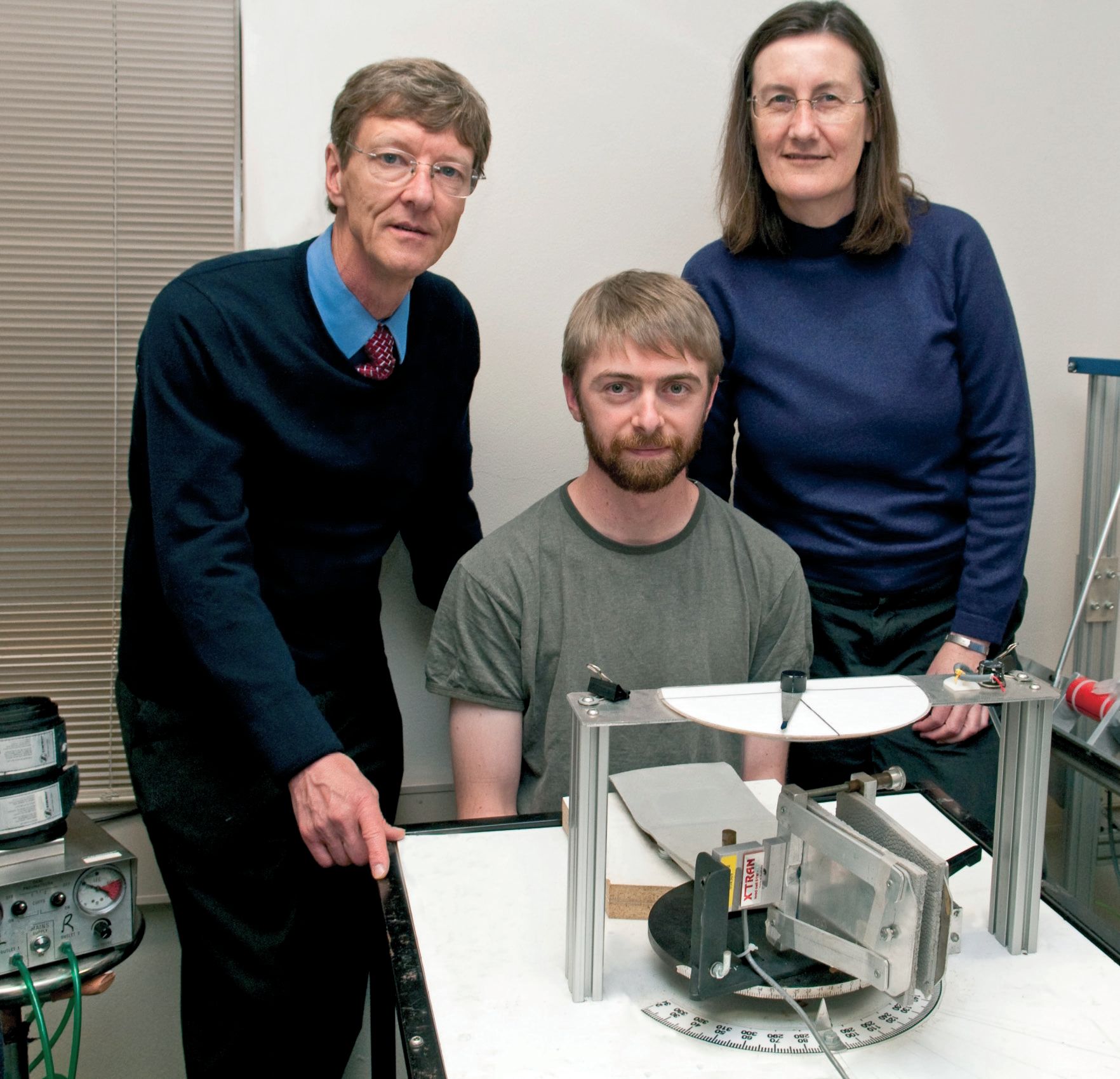
It is possible to induce temporary experimental phantoms in human subjects. A simple way of doing this for the wrist and hand is to inflate a blood pressure cuff around the upper arm (Fig. 1). This cuts off the blood supply to the arm below the cuff. After 30-40 min the arm will become anaesthetised and paralysed. Motor nerves cannot conduct commands to move the arm below the cuff and no sensory information is generated. Despite the lack of sensory information, subjects continue to experience the presence of their anaesthetised and paralysed forearm, wrist and hand. That is, they experience a phantom. We used this method in a recent study (Walsh et al. 2010) and once subjects were paralysed and anaesthetised we asked them to make efforts to flex or extend their wrist and to indicate what happened with a pointer. The subjects indicated that when they made voluntary efforts their phantom wrists moved in the same direction as their effort. If they made an effort into flexion with their wrist they perceived their wrist to move into flexion, despite the muscles being paralysed. This sensation was one of continuous movement rather than of the phantom simply adopting a new position. If subjects made longer-duration efforts their phantom wrist moved further and if they made stronger efforts it moved further and faster (Fig. 2).

When we make movements the brain generates central signals that ultimately result in motoneurone output to the appropriate muscles, causing them to contract and move the limb. These signals are commonly referred to as ‘motor commands’ and our results suggest that information about these commands is used to update the body schema regarding limb movements. There is no sensory information coming back to the brain from the anaesthetised wrist, but while the motor commands cannot get to the paralysed wrist muscles to activate them, they are still generated by the brain and presumably still undergo their normal processing. The idea is that when the brain generates a motor command, information about which muscles are activated, how strongly they are activated, and for how long, is used to update the body schema. This information can give an accurate indication of the movement if there are no unexpected events. This mechanism is likely to be the one that allows amputees to move their phantom limbs.
It is not a new idea that information about motor commands could be used to tell us about the position and movements of our limbs. However, the idea was unpopular for most of the last century, despite the dominance of these signals in oculomotor theory (e.g. Donaldson, 2000). Over the last few years, however, there has been increasing evidence that these signals which originate in the brain combine with those sensory signals from the limbs to give us our complete senses of limb position and movement. The big question now is how do these two classes of signals interact with each other to produce these critical senses that we rely on every time we make a movement.
References
Cole J (1991). Pride and a Daily Marathon. Gerald Duckworth & Co., London.
Collins DF, Refshauge KM, Todd G & Gandevia SC (2005). Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94, 1699–1706.
Donaldson IM (2000). The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci 355, 1685–1754.
Goodwin GM, McCloskey DI & Matthews PBC (1972). The contribution of muscle afferents to kinæsthesia shown by vibration induced illusion of movement and by the effects of paralysing joint afferents. Brain 95, 705–748.
Reilly KT, Mercier C, Schieber MH, Sirigu A (2006). Persistent hand motor commands in the amputees’ brain. Brain 129, 2211–2223.
Walsh LD, Gandevia SC & Taylor JL (2010). Illusory movements of a phantom hand grade with the duration and magnitude of motor commands. J Physiol 588, 1269–1280. http://jp.physoc.org/content/588/8/1269.long