
Physiology News Magazine
Generation of complex neuronal behaviour in a mammalian nervous system
Our understanding of motor patterns within mammalian neuronal networks has been limited by our ability to record activity in a large number of neurons simultaneously. We have used imaging techniques to explore a relatively simple mammalian neural network, the enteric nervous system, to determine how activity in different classes of neurons produces a complex motor behaviour.
Features
Generation of complex neuronal behaviour in a mammalian nervous system
Our understanding of motor patterns within mammalian neuronal networks has been limited by our ability to record activity in a large number of neurons simultaneously. We have used imaging techniques to explore a relatively simple mammalian neural network, the enteric nervous system, to determine how activity in different classes of neurons produces a complex motor behaviour.
Features
Peter O Bayguinov, Grant W Hennig amd Terence K Smith
Department of Physiology and Cell Biology, University of Nevada School of Medicine, Reno, Nevada, USA.
https://doi.org/10.36866/pn.80.29

The functional properties of a nervous system are largely determined by activity in individual neurons and the synaptic connections they receive or make on their targets. Networks of neurons that make synaptic connections with each other have emergent properties that aren’t normally predictable by studying the characteristics of single neurons. Much of what is known regarding the function of neurons has been largely the result of studies of individual cells. Most studies have been restricted to invertebrates due to the relatively low number of neurons present, and their conserved neuronal topography, allowing for easy identification of the cells. Despite the small number of neurons, studies of invertebrate nervous systems have yielded insight into the functional basis of more complex motor events, such as swimming behaviour, rhythmic breathing and oscillatory activities such as chewing and locomotion. Similar studies on mammalian systems have been difficult due to the sheer number of neurons involved, their looser topography and the distance between neuron cell bodies and the targets they innervate. Studies in cortical slices cannot monitor physiological inputs or motor outputs.
The enteric nervous system (ENS) (Langley, 1900), or ‘little brain’, in mammals is one of the three subdivisions of the autonomic nervous system, innervating the alimentary canal, along with the gall bladder, pancreas and the bile duct. From a neuroscientist viewpoint, it is an intriguing system to study, as it is the only large collection of neurons outside of the CNS that is capable of generating its own reflexes and motor patterns. Neurons in the ENS are organized in a nearly two-dimensional manner in two well-defined plexuses, called the myenteric and submucous plexus. The myenteric plexus, which mainly regulates motility, is situated between the longitudinal and circular muscle layers, whereas the submucous plexus, which regulates secretion of water and electrolytes, lies in the submucosa close to the circular muscle. The complexity of the ENS can be appreciated by the fact that it contains approximately 108 neurons, roughly the same amount as in the spinal cord. Myenteric neurons in the large bowel form interconnected ganglia (roughly 1600 per cm2), and each ganglion contains between 40 and 120 neurons, including sensory neurons, interneurons, and excitatory and inhibitory motoneurons with no obvious topography within each ganglia (Fig. 1A).
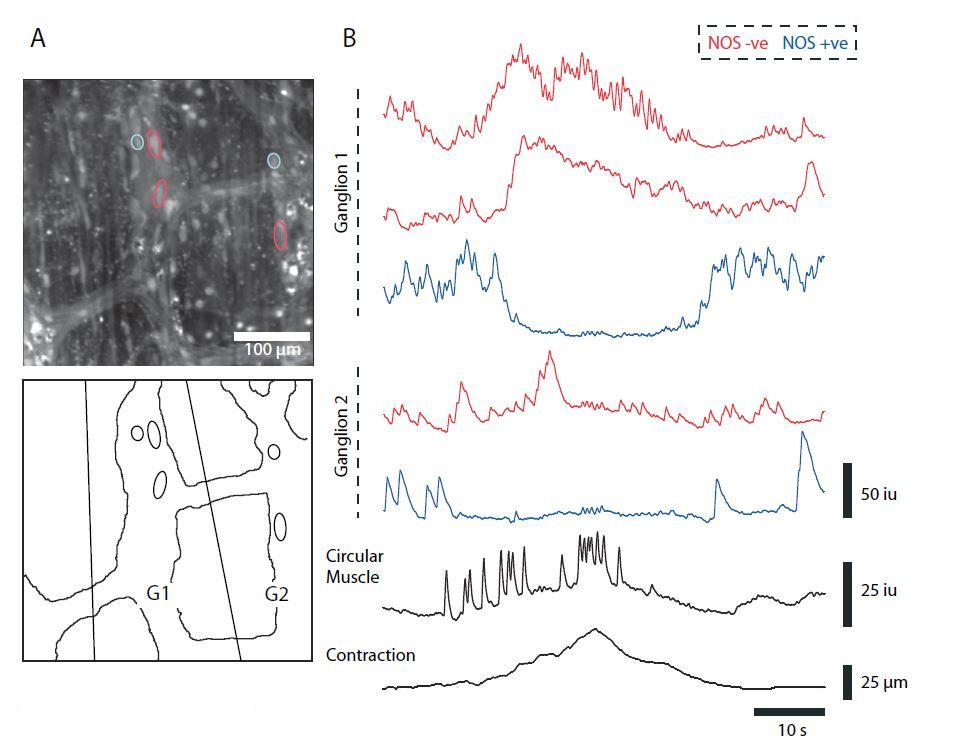
Slow and fast synaptic events in myenteric neurons can give rise to action potentials that generate robust and prolonged calcium transients that can be readily monitored using indicators such as Fluo-4. As Ca2+ is ubiquitous in all excitable cells, determining the chemical coding of individual neurons requires post hoc labelling. In a recent study (Bayguinov et al. 2010), we used Ca2+ imaging in conjunction with immunohistochemical staining to report the activity of myenteric neurons simultaneously from several ganglia in the ENS, as well as the longitudinal and circular muscle, during the colonic migrating motor complex (CMMC) that would be impossible using traditional electrophysiological methods. The CMMC is a rhythmic, neurally mediated motor pattern in the colon which has been shown to underlie fecal pellet propulsion in mice (Heredia et al. 2009). To differentiate between the two major classes of motoneurons, we used NOS post hoc labelling to label inhibitory motoneurons.
In our recordings, we observed ongoing Ca2+ transient activity in many NOS+ (nitrergic) and NOS– (cholinergic) Dogiel Type I myenteric unipolar neurons between the CMMC, yet there was little coordination of this activity among neurons in the same ganglion, or among neurons in different ganglia. NOS+ neurons often exhibited rhythmic bursts of activity, that were probably responsible for mediating the tonic inhibitory input to the colonic muscle. At the onset of the CMMC, there was a synchronous activation of many NOS– myenteric neurons (Fig. 1B, red traces), some of which remained active for the duration of the CMMC, suggesting that they were excitatory motoneurons that release ACh and tachykinins (TK) onto the muscle. This activation coincided with increases in Ca2+ transient activity in both the circular (Fig. 1B) and longitudinal muscle layers, leading to muscle contractions. Activity in a proportion of NOS+ neurons that exhibited rhythmic activity between CMMCs ceased just after the onset of the CMMC (Fig. 1B) and often remained quiescent even after the termination of the CMMC. This suggests that activation of excitatory motoneurons, together with a suppression of activity of NOS+ inhibitory motoneurons is necessary for the full and synchronous activation of both muscle layers during a CMMC.
Mechanical stimulation of the mucosa, applied at long distances away from the recording site (at either end of the colon) evoked CMMCs, as visualized by a burst of Ca2+ transients in the muscle. Evoked CMMCs were not only similar in duration to spontaneous CMMCs, but also led to similar responses in the same myenteric neurons, with NOS+ neurons becoming quiescent, and NOS– neurons increasing their activity. Moreover, mucosal stimulations at either end of the preparation evoked prolonged Ca2+ transient activity in multipolar Dogiel Type II neurons, which have projections to the mucosa, and are considered to be a major sensory neuron in the ENS. The fact that both oral and anal stimulation of the mucosa excited or inhibited the same myenteric neurons suggests that there is considerable convergence of interneuronal pathways onto common motoneurons.
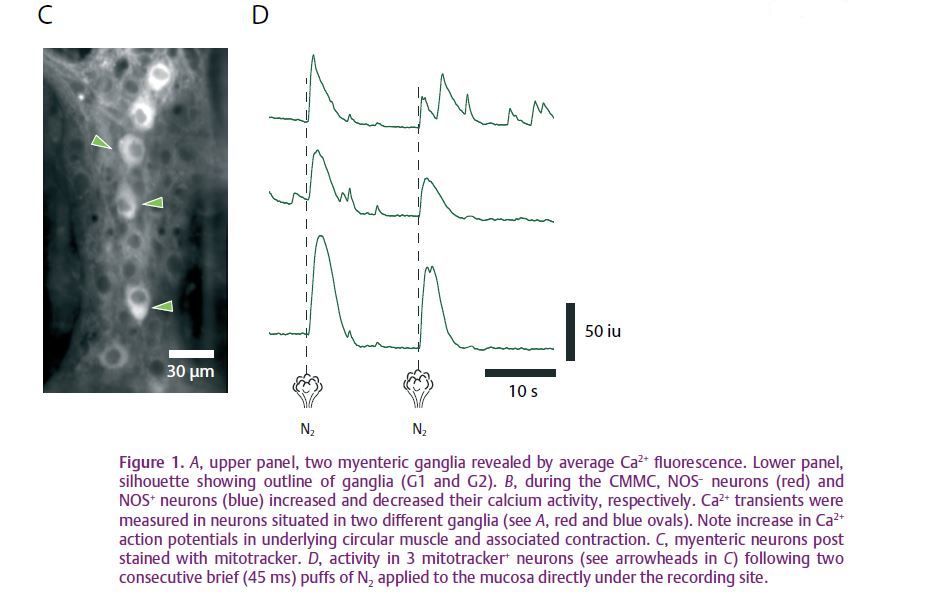
We were interested in examining the responses of myenteric neurons at the site of stimulation, and, in particular, the activity of Dogiel Type II neurons, as these neurons were previously thought to initiate peristaltic reflexes but not the CMMC. Type II neurons were readily identifiable using mitotracker dyes (Fig. 1C), since they, unlike other myenteric neurons, contain dense mitochondria. We found that Type II neurons appeared to be the first responders to local mechanical stimulation of the mucosa with a brush directly over the recording site, or a puff of N2 applied directly to the mucosa under the recording site (Fig. 1D). Following several consecutive stimulations with a brush, there was a coordinated build-up of activity in these neurons, which led to the eventual initiation of a CMMC. Application of ondansetron (a 5-HT3 receptor antagonist) greatly reduced these responses in Type II neurons, suggesting that they were being activated by 5-HT released from mechanosensitive enterochromaffin (EC) cells in the mucosa.
When the mucosa was removed from the colon, activity in myenteric neurons did not appear to be affected, as we observed similar ongoing Ca2+ transients in both NOS+ and NOS– neurons, including Dogiel Type II neurons. Removal of the mucosa abolished the CMMC, and we failed to observe a single CMMC in 35 colonic preparations. Although the neural circuitry underlying the CMMC appears to behave like a central pattern generator, as seen in invertebrates, input from the mucosa appears to be essential for bringing Type II neurons to threshold to initiate this motor pattern.
Unlike the peristaltic reflex (Bayliss & Starling, 1899), which is graded according to stimulus strength and conducts rapidly through the myenteric plexus, the CMMC appears to be an all-or-none event that propagates relatively slowly (0.8 mm s–1) and probably requires the activation of Dogiel Type II neurons at different points along the colon for its regeneration (Fig. 2).

This study presents an important step in our understanding of how an integrated neural network in a mammalian nervous system functions to generate a complex rhythmic motor behaviour.
Acknowledgements
This research was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (USA): RO1 DK45713. Calcium imaging was performed in a Core facility funded by NIH grant P20 RR-1875.
References
Bayguinov PO, Hennig GW & Smith TK (2010). Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol 588, 399–421. http://jp.physoc.org/content/588/3/399.long
Bayliss WM & Starling EH (1899). The movements and innervation of the small intestine. J Physiol 24, 99–143.
Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW & Smith TK (2009). Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136, 1328–1338.
Langley JN (1900). The sympathetic and other related systems of nerves. In Text-Book of Physiology, ed. Schäfer E, pp. 616–696. Pentland, Edinburgh.
