
Physiology News Magazine
‘Sensible to feeling as to sight’(1) Some recent progress on mechanosensory transduction in mammals
Our understanding of the molecular basis of sensory excitation by mechanical stimulation in mammals remains rudimentary, despite its fundamental role in touch sensation, and blood pressure and movement control. But recent advances in the little roundworm, Caenorhabditis elegans, have provided clues that have led to evidence for a surprising candidate for a possible component of the transducer.
Features
‘Sensible to feeling as to sight’(1) Some recent progress on mechanosensory transduction in mammals
Our understanding of the molecular basis of sensory excitation by mechanical stimulation in mammals remains rudimentary, despite its fundamental role in touch sensation, and blood pressure and movement control. But recent advances in the little roundworm, Caenorhabditis elegans, have provided clues that have led to evidence for a surprising candidate for a possible component of the transducer.
Features
Guy Bewick (1) and Bob Banks (2)
1: School of Medical Sciences, University of Aberdeen, Aberdeen AB25 2ZD, UK
2: School of Biological & Biomedical Sciences, University of Durham, Durham DH1 3LE, UK
1: Macbeth Act 2, scene 1.
https://doi.org/10.36866/pn.80.33

In 1993 Canessa, Horisberger and Rossier published a letter to Nature entitled ‘Epithelial sodium channel related to proteins involved in neurodegeneration’. Although there is no hint of this in the title, the paper later proved to be a spur to research in one of the most intractable problems of mammalian sensory neuroscience: that of the mechanism by which neurons respond to touch, muscle stretch, blood pressure and similar mechanical stimuli. Canessa et al. (1993) had isolated and cloned a sequence from a rat colon cDNA library that hybridized with transcripts from various Na+-transporting epithelia. When they injected Xenopus oocytes with cRNA, the cells expressed an amiloride-sensitive Na+ current that was absent from controls. And when they searched databases for proteins with similar amino acid sequences to their newly discovered Na+ channel, which they called αrENac, Canessa et al. came up with two members of the degenerin family of Caenorhabditis elegans proteins: deg-1 and mec-4. The degenerins had been found by genetic screening for mutants of C. elegans that are insensitive to touch (Driscoll & Chalfie, 1992). They had been only partially cloned, so while their function was unknown, specific mutations of them caused certain mechanosensory neurons to degenerate during development. Canessa et al. (1993) proposed that the degenerins and ENac belong to the same protein family (now generally called DEG/ENaC) consisting of ion channels involved in intra- and extracellular volume control, and that might also include mechanosensitive ion channels.
The DEG/ENaC family continued to enlarge with the addition of the molluscan FMRFamide receptor, Ripped Pocket (RPK) and Pickpocket (PPK) from Drosophila melanogaster and the mammalian acid-sensing ion channels (ASICs, also known as brain sodium channels, BNC or BNaC), as well as further ENaC subunits. All are characterised as contributing to non-voltage-dependent, amiloride-sensitive ion channels selective for Na+ (Kellenberger & Schild, 2002). Meanwhile the cross-fertilization engendered by the original union of the degenerins and ENaCs in one family was beginning to produce mechanosensory fruit. To date this has developed best on the nematode branch of the phylogenetic tree, where early indications that mec-4 and the related mec-10 might constitute a Na+ channel as part of a multi-protein complex responsible for detecting light touch have been borne out (O’Hagan et al. 2005). Reciprocally, the recognition of the likely involvement of degenerins in nematode mechanotransduction soon led to suggestions that the ENaCs, and later the acid-sensing ion channels (ASICs), might be similarly important in mammals.
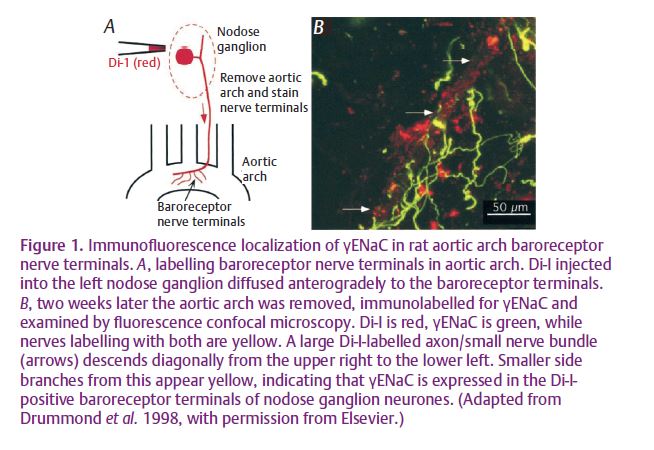
In the first such study, Drummond et al. (1998) used RT-PCR to demonstrate that b- and g-ENaCs are expressed in the nodose ganglion of rat, which contains neurons that supply baroreceptor endings to the aortic arch. Although the pore-forming α-subunit was not found by them, Drummond et al. did find that amiloride inhibited the response of the ganglion cells to mechanical stimulation (assessed as a rise in intracellular [Ca2+]), and that benzamil, an amiloride analogue, reduced both the response of rabbit carotid sinus baroreceptors to blood pressure and the resulting pressor reflex. In addition, they used immunocytochemistry to localise γ-ENaC to the sensory endings and ganglion cells (Fig. 1). A similar study, also by Drummond et al. (2000), on rat cervical and lumbar dorsal root ganglion cells came to similar conclusions, but in this case the techniques were confined to RT-PCR and immunocytoche Antibodies against both b- and y-ENaCs were used to localise the proteins to the sensory endings of Merkel-cell afferents (though not the Merkel cells themselves), Meissner and lamellated corpuscles of the foot pad. At the same time, and using the same techniques, Fricke et al. (2000) reported the presence of α-, – and -ENaCs, and stomatin in rat trigeminal ganglion cells and the lanceolate terminals of vibrissal sinus hair follicles, innervated by the trigeminal ganglion. Stomatin is homologous to mec-2, which is thought to be important in linking the mec-4/mec-10 ion channel to the cytoskeleton in C. elegans.
The ASICs are another branch of the DEG/ENaC family, distinct from both the degenerins and the ENaCs. Price et al. (2000) generated a mouse with a BNC1 (ASIC2) null mutation by homologous recombination to disrupt the gene. Then, using an in vitro skin/nerve preparation, they found that both rapidly and slowly adapting mechanoreceptors showed reduced responses to defined stimuli. In other respects, the phenotypes of the homozygous mutant mice appeared quite normal. By immunocytochemistry, Price et al. localised BNC1 (ASIC2) to hair follicle sensory endings, particularly the lanceolate terminals of palisade endings; shortly afterwards, Garcia-Añoveros et al. (2001) demonstrated that the splice variant BNaC1α is targeted to the peripheral, but not the central, projections of dorsal-root ganglion cells where it was localised in the sensory terminals of several types of mechanoreceptor in glabrous and hairy skin.
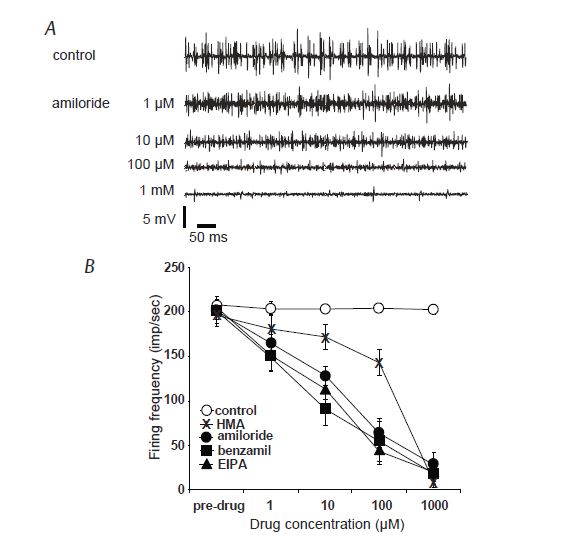
For ten or so years now, we have been working on transduction in the muscle spindle, that most complex of mammalian mechanosensory receptors (after the ear). The muscle spindle has a special place in sensory and synaptic physiology due, primarily no doubt, to its innervation by the largest afferent axons in the body and their monosynaptic connexions with motoneurons in the neuraxis, but also to the relative ease and precision with which stimulation by muscle stretch may be delivered to it. The sensory terminals of muscle spindles are, of course, the peripheral endings of dorsal-root ganglion (DRG) cells and so might be expected to share some functional properties with other mechanosensory DRG cells. The recent paper from our group in The Journal of Physiology (Simon et al. 2010) lends support to this hypothetical similarity of function. There we show that amiloride and several of its analogues can greatly inhibit, or even completely block, the sensory response of the muscle spindle to a maintained stretch, and that this is unlikely to be due to effects on action potential transmission (Fig. 2). We also present biochemical evidence for the expression of at least three of the ENaC subunits – α, β and γ – in muscle spindles and immunocytochemical evidence for the localisation of ASIC2 and the ENaC subunits in the sensory terminals.

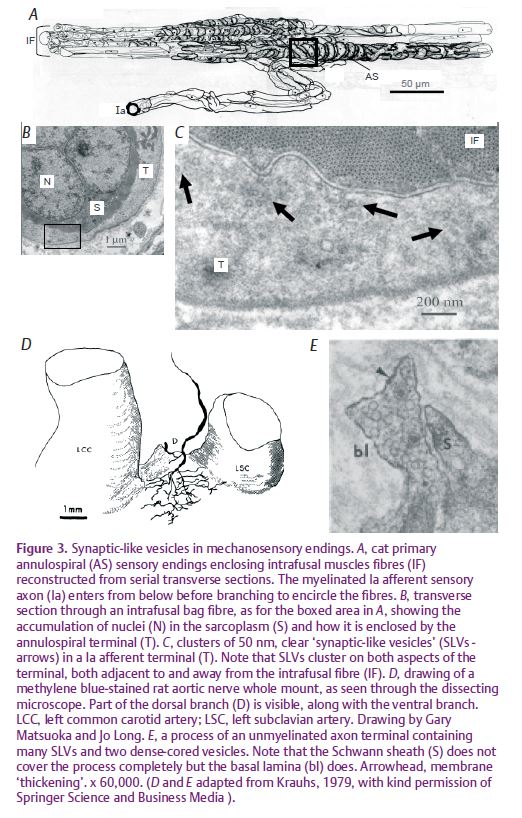
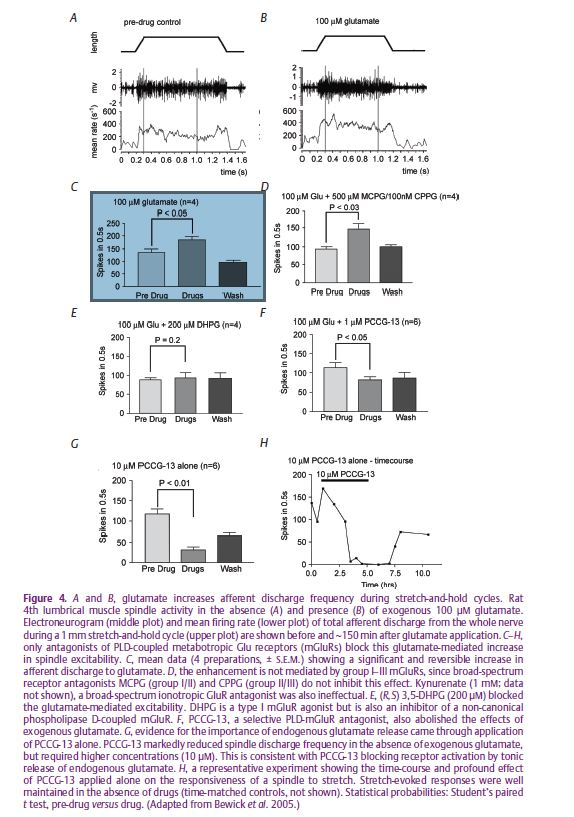
This much, then, is consistent with the earlier studies implicating members of the DEG/ENaC family in mechanotransduction by touch- and baroreceptors. But we also showed that ASIC2 and the ENaCs are colocalised with synaptophysin in the spindle’s sensory terminals, so linking the possible primary events of mechanotransduction with the system of ‘synaptic-like vesicles’ (SLVs), previously described by us (Bewick et al. 2005). SLVs are certainly not unique to muscle spindles (Fig. 3), and may be a common feature of mechanosensory endings, at least those of primary afferent neurons. In our use of the spindle as a model for this system we (Bewick et al. 2005) showed that the SLVs appear to be part of a glutamatergic mechanism for the auto-regulation or modulation of the sensory terminals’ excitability. The SLVs recycle to and from the terminal membrane in an activity-dependent manner, both exo- and endocytosis being Ca2+ dependent, and probably release glutamate which acts via a non-canonical phospholipase-D-linked metabotropic glutamate receptor (mGluR) to maintain, or increase, the excitability of the terminals themselves (Fig. 4). The mechanism is of fundamental importance to mechanotransduction, since specific antagonism of the mGluR by the glycine derivative PCCG-13 can reversibly silence the output of the sensory ending, possibly as a result of receptor-mediated inactivation of the primary mechanotransducer (a protein complex possibly including ASIC2/ENaC) at the terminal membrane, or its sequestration within the terminal in the membranes of the SLVs.
Perhaps the main reason the molecular basis of mechanosensation has been so elusive in all its forms in animals, and especially mammals, is its unlooked-for complexity. What we have briefly described here may well prove to be an important part of the story, but it will certainly not be the whole story. Thus, whereas DEG/ENaCs may constitute an important ion channel, other ion channels from other protein families (transient receptor potential, or TRP, channels perhaps) are also likely to be important in at least some sensory endings. And, even if the principal ion channels and some aspects of their functional expression have been identified, their gating mechanism remains essentially unknown. Exciting times, indeed!
References
Bewick GS, Reid B, Richardson C & Banks RW (2005). Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol 562, 381–394.
Canessa CM, Horisberger J-D & Rossier BC (1993). Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 361, 467–470.
Driscoll M & Chalfie M (1992). Developmental and abnormal cell death in C. elegans. Trends Neurosci 15, 15–19.
Drummond HA, Abboud FM & Welsh MJ (2000). Localization of b and g subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res 884, 1–12.
Drummond HA, Price MP, Welsh MJ & Abboud FM (1998). A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21, 1435–1441.
Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M & von Düring M (2000). Epithelial Na+ channels and stomatin are expressed in rat trigeminal mechanosensory neurons. Cell Tissue Res 299, 327–334.
Garcia-Añoveros J, Samad TA, Žuvela-Jelaska L, Woolf CJ, & Corey DP (2001). Transport and localization of the DEG/ENaC ion channel BNaC1α to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci 21, 2678–2686.
Kellenberger S & Schild L (2002). Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82, 735–767.
Krauhs JM (1979). Structure of rat aortic baroreceptors and their relationship to connective tissue. J Neurocytol 8, 401–414.
Li Q, Lau A, Morris TJ, Guo L, Fordyce CB & Stanley EF (2004). A syntaxin 1, Gαo, N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24, 4070–4081.
O’Hagan R, Chalfie M & Goodman MB (2005). The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8, 43–50.
Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA et al. (2000). The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407, 1007–1011.
Simon A, Shenton F, Hunter I, Banks RW & Bewick GS (2010). Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588, 171–185. http://jp.physoc.org/content/588/1/171.full
