
Physiology News Magazine
Excitation-dependent Ca2+ influx in vertebrate skeletal muscle is in, again
An influx of extracellular Ca2+ into skeletal muscle excited by action potentials was originally reported in the 1950s. At least three distinct Ca2+ entry pathways across the transverse tubular system of working skeletal muscle have since been identified. The recently discovered action potential-activated current appears to be the pathway responsible for the excitation-dependent Ca2+ influx but its specific physiological roles remain speculative
Features
Excitation-dependent Ca2+ influx in vertebrate skeletal muscle is in, again
An influx of extracellular Ca2+ into skeletal muscle excited by action potentials was originally reported in the 1950s. At least three distinct Ca2+ entry pathways across the transverse tubular system of working skeletal muscle have since been identified. The recently discovered action potential-activated current appears to be the pathway responsible for the excitation-dependent Ca2+ influx but its specific physiological roles remain speculative
Features
Bradley S. Launikonis (1), Oliver Friedrich (1) and D. George Stephenson (2)
1: School of Biomedical Sciences, The University of Queensland, Brisbane, Qld, Australia
2: Dept of Zoology, La Trobe University, Melbourne, Vic, Australia
https://doi.org/10.36866/pn.77.20

Skeletal muscle cells in vertebrates, when compared with cardiac muscle cells, evolved to be able to deliver calcium for the activation of the contractile apparatus in a faster and more precise manner, in order to suit completely different functions in the body. In cardiac cells there is a fundamental need for Ca2+ influx to trigger the cascade of events that lead to contraction in the process known as excitation–contraction coupling (EC coupling; Bers, 2001). In contrast, work from Lüttgau’s laboratory in the 1970s showed unequivocally that skeletal muscle could twitch and develop tetanic force in the absence of extracellular Ca2+. This represented a major deviation from the physiology of the cardiac cell. Nevertheless, Bianchi & Shanes showed in 1959 that an infl ux of Ca2+ into skeletal muscle occurred during excitation by measuring 45Ca2+ entering a muscle after a couple of hours of low-frequency stimulation. This observation was confirmed by others over the next three to four decades, but a precise role of this excitation-dependent Ca2+ entry in skeletal muscle cells was not defined by Bianchi & Shanes or the other researchers. Although no argument can be made for a specific requirement of extracellular Ca2+ to produce force in skeletal muscle, if one were to look closely at some of the force traces presented in the work published by Lüttgau & Spiecker (1979), one could notice that a smaller initial force level was achieved at the beginning of a tetanus in the absence compared to the presence of extracellular Ca2+.
At least three distinct Ca2+ entry pathways are known across the transverse tubular (t) system membrane of skeletal muscle fi bres that could be functional during periods of work and responsible for the influx described by Bianchi & Shanes (1959; Fig. 1).
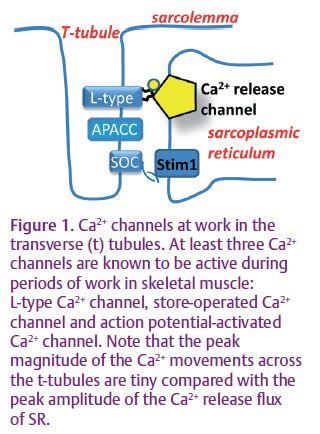
The L-type Ca2+ channel is activated during square depolarizing pulses. The resulting slow, inward Ca2+ current is easily detected with conventional electrophysiological techniques (Melzer et al. 1995) and this is perhaps the reason that this current is so well described, at least biophysically. It has been assumed that a high frequency of action potentials in skeletal muscle may be equivalent to chronic depolarization, to induce Ca2+ influx through the L-type channel. However, experimental evidence for this in adult mammalian skeletal muscle is hard to find. Indeed, chronic depolarization and a high frequency of action potentials induce very different electrical waveforms across the t-system, highlighting the need for the current to be examined under more physiological conditions. This makes it most unlikely for the L-type Ca2+ current to be responsible for the Ca2+ infl ux measured in the experiments of Bianchi & Shanes.
Furthermore, a chronic depolarization of muscle in the presence of the specific L-type channel blocker nifidepine only reduces the Ca2+ influx by a small amount when the SR Ca2+ is low (Kurebayashi & Ogawa, 2001). This means that other, more signifi cant Ca2+ infl ux pathway(s) are activated during a depolarization when the SR Ca2+ is low. In their paper, Kurebayashi & Ogawa describe a store-dependent Ca2+ infl ux into skeletal muscle. Using chronic depolarization via ionic substitution of the external bathing solution, they show that most of the Ca2+ in the SR needs to clear SR in order to induce the store-dependent current. In non-excitable cells Ca2+ must be lost from the cell to induce store-operated Ca2+ entry (SOCE). However, in skeletal muscle this is not likely to occur physiologically. In skeletal muscle, Ca2+ within SR needs only to drop to levels that cause Ca2+ dissociation from the Ca2+-sensing protein, Stim1, located in the SR membrane (Fig. 1). Ca2+ released from the SR during muscle contraction binds to cytoplasmic buffering sites, causing transient Ca2+ depletion of the SR. Thus, a situation can occur during periods of intense muscle work when large amounts of calcium are bound to cytoplasmic Ca2+ binding sites causing sufficient SR Ca2+ depletion to activate SOCE.
In skeletal muscle SOCE is activated across the t-system (Fig. 1), providing a proximal source of Ca2+ for the closely apposed and depleted SR (Launikonis & Ríos, 2007). Thus, extended periods of stimulation can induce a physiologically important entry of Ca2+ in muscle. However, this still does not provide an explanation for the results of Bianchi & Shanes (1959).
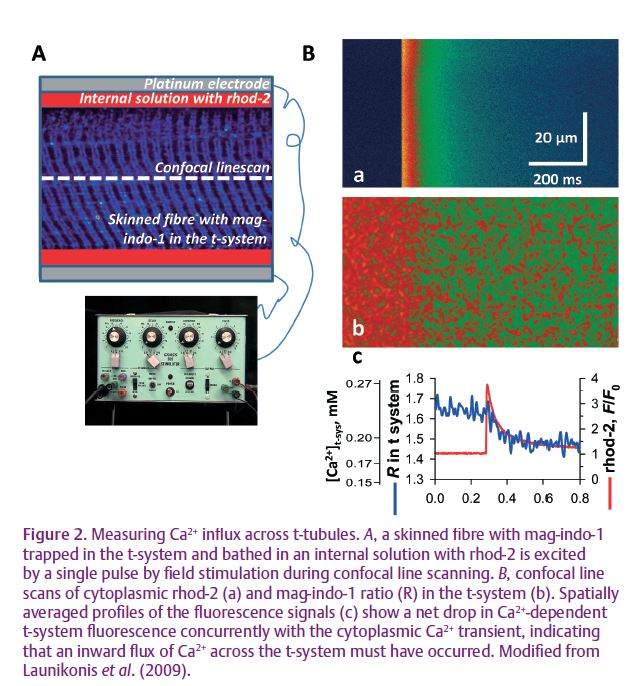
No fast Ca2+ influx activated by the equivalent of a brief action potential has been reported in adult mammalian skeletal muscle using conventional electrophysiological techniques. Recently, a novel fluorescence technique has allowed the imaging of Ca2+ transients simultaneously within the lumen
of the sealed t-system and open cytoplasmic environment of mechanically skinned fibres from rat (Fig 2). In this preparation the sealed t-system is able to repolarise in a suitable cytoplasmic environment and develop action potentials when stimulated by a field pulse. An action potential-activated Ca2+ current (APACC) was observed for the first time using this novel approach, concurrent with the cytoplasmic Ca2+ transient (Launikonis et al. 2009). The physical properties of this current are consistent with an influx of Ca2+ following each action potential at a sustained low frequency of stimulation or the initial two or three action potentials in a train, with a short rest period in between. APACC has all the major characteristics of the Ca2+ influx observed by Bianchi & Shanes (1959) and others, strongly suggesting that the excitation-dependent Ca2+ influx occurring in the adult skeletal muscle is via APACC.
What then, would be the physiological role of APACC? Currently, we can only speculate on this. The small, action potential-dependent Ca2+ influx may raise the [Ca2+] in the vicinity of the ryanodine receptor before it is first activated by the voltage sensors at the beginning of a tetanus, thus helping the ryanodine receptor to open more fully when it becomes activated. This would also explain the smaller initial force level achieved at the beginning of a tetanus in the absence compared to the presence of extracellular Ca2+ in the experiments of Lüttgau & Spiecker (1979). The APACC will also cause a transient depletion of Ca2+ within the transverse tubules. This depletion may activate extracellular Ca2+ sensors involved in monitoring muscle activity. These and other possibilities remain to be explored. However, it is clear that the excitation-dependent Ca2+ influx in skeletal muscle has returned to the limelight.
References
Bers DM (2001). Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn, Kluwer Academic Publishers.
Bianchi CP & Shanes AM (1959). Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol 42, 803–815.
Kurebayashi N & Ogawa Y (2001). Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol 533, 185–199.
Launikonis BS & Ríos E (2007). Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol 583, 81–97.
Launikonis BS, Stephenson DG & Friedrich O (2009). Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol 587, 2299–3312. http://jp.physoc.org/content/587/10/2299. long
Lüttgau HC & Spiecker W (1979). The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres from the frog. J Physiol 296, 411–429.
Melzer W, Herrmann-Frank A & Lüttgau HC (1995). The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1241, 59–116.
