
Physiology News Magazine
Regulation of extracellular pH by purinergic signalling
Little is known about extracellular pH regulation, despite its importance for proper organ functioning. We present data supporting a hypothesis that alkaline phosphatase, through pH-dependent ATP hydrolysis, serves as an extracellular pH sensor. Dysregulation of extracellular pH regulation may help explain the tissue dysfunction characteristic of diseases such as cystic fibrosis
Features
Regulation of extracellular pH by purinergic signalling
Little is known about extracellular pH regulation, despite its importance for proper organ functioning. We present data supporting a hypothesis that alkaline phosphatase, through pH-dependent ATP hydrolysis, serves as an extracellular pH sensor. Dysregulation of extracellular pH regulation may help explain the tissue dysfunction characteristic of diseases such as cystic fibrosis
Features
Jonathan D Kaunitz (1,2,3) and Yasutada Akiba (1,2,3)
1: Greater Los Angles Veterans Affairs Healthcare System
2: Department of Medicine, School of Medicine, University of California Los Angeles
3: Brentwood Biomedical Research Institute, Los Angeles, CA 90073, USA
https://doi.org/10.36866/pn.77.22

Enzymes function best in a narrow pH range. The pH of the intracellular environment is regulated to within 0.1 pH unit (10–8 M [H+]) by well-described plasma membrane acid–base transporters, although the actual intracellular pH sensor is not known. While many important enzymes have extracellular catalytic sites (ecto-enzymes), less is known about the regulation of extracellular pH (pHo) despite its importance for a variety of cellular processes. Of particular interest is pHo regulation in compartments which are thought to have pH far from neutrality, whether acidic in the activated parietal cell canaliculus or osteoclast lacuna, or alkaline at the proximal duodenal surface, the pancreatic duct, salivary gland or the oviduct.
Several ecto-enzymes have strongly acidic and alkaline pH optima, such as the acid and alkaline phosphatases. Although these unusual optima were described decades ago, the physiological significance of such unusual conditions has been largely unknown. Nevertheless, the expression patterns of these enzymes provide valuable clues as to their possible function. For example, acid phosphatases are expressed in the aforementioned acid-secreting organs and also on acidic organelles such as lysosomes, whereas alkaline phosphatase is expressed in the alkali-secreting tissues mentioned above. Importantly, both alkaline phosphatase and acid phosphatase hydrolyse purine nucleotides, in addition to the other organic and inorganic phosphates for which they have hydrolytic activity.
ATP and other purine nucleotides serve as neurotransmitters as part of a purinergic signalling system. Components of the system include exocytotic release of ATP from subsurface granules, interaction with well-defined purinergic G-protein-coupled cell surface receptors or ionotropic receptors, and hydrolysis by ecto-hydrolases. Although purinergic neurotransmission was initially described in the 1970s, the concept that extracellular ATP is an important non-neuronal regulatory molecule is relatively recent (Leipziger, 2003; Burnstock, 2007). Particularly in secretory epithelia, extracellular ATP increases anion or HCO3– secretion through interaction with P2Y receptors. Indeed, selective P2Y agonists have been developed to overcome the airway anion secretory impairment characteristic of the disease cystic fibrosis (CF).
Though intuitively wasteful, cellular regulation by extracellular ATP does have the attraction of a signalling molecule present in millimolar concentrations in the cytosol, numerous sensitive and selective cell-surface receptors, and potent ecto-hydrolases such as alkaline phosphatase and ecto-nucleotide phosphohydrolase (ENTPDase or CD39). Nevertheless, the mechanism of epithelial non-lytic ATP release is not well understood, nor is the function of many other membrane proteins and ecto-enzymes that are probably involved.
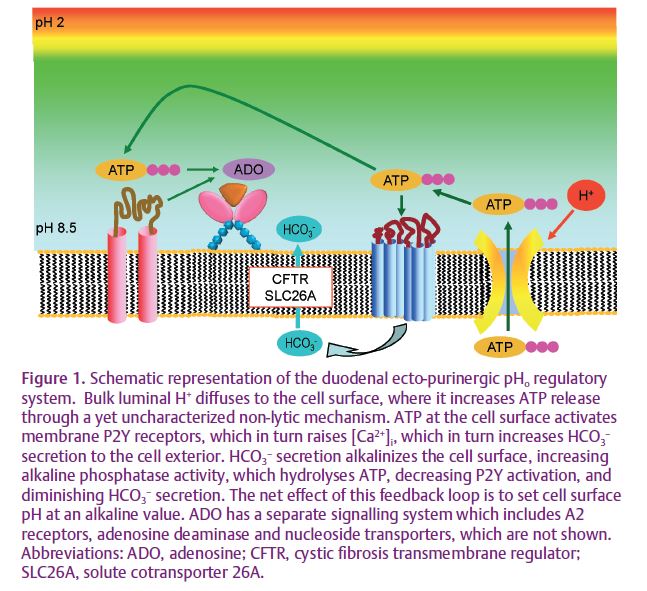
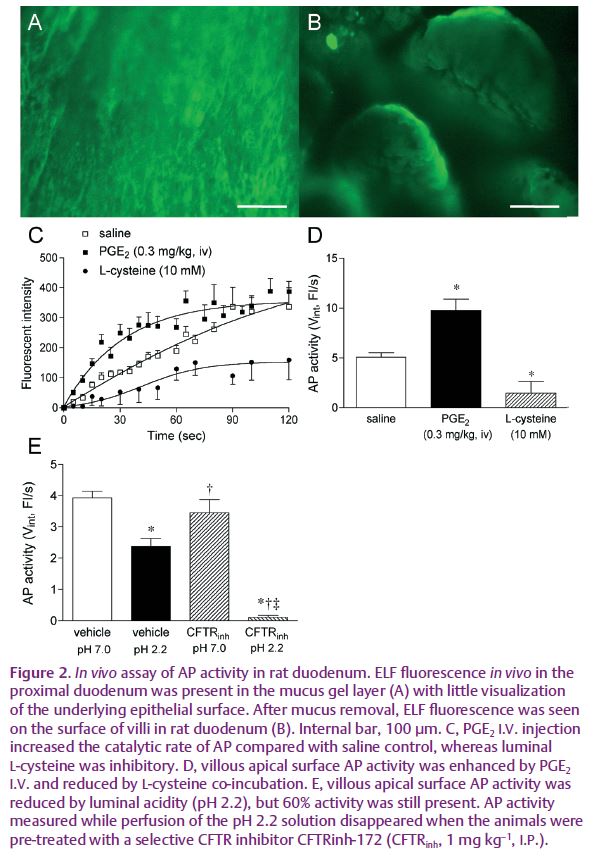
We have recently published data supporting a hypothesis wherein ecto-phosphohydrolases with extreme pH optima serve as pHo sensors in alkaline or acidic compartments. We hypothesized that since activation of P2Y receptors increases alkaline secretion into a small compartment, the pH-sensitive hydrolytic activity of alkaline phosphatase (AP) is dependent on local pH, which in turn is set by the rate of alkaline secretion. Thus, for an alkaline compartment, a high rate of alkaline secretion increases the hydrolytic activity of AP, decreasing extracellular ATP concentration ([ATP]o), and decreasing the rate of alkaline secretion, thus serving as a negative feedback loop. In this fashion, pHo is regulated by pH-sensitive ATP hydrolytic activity of AP (Fig. 1). To test this hypothesis, we have studied the rat proximal duodenum, the dimensions of which (~5 mm diameter), combined with its accessibility, provide a convenient live-animal system. With this system we have reported that the rat proximal duodenal brush border expresses P2Y1 receptors, AP and ENTPDase, and that exogenous ATP augments the rate of epithelial HCO3– secretion (Akiba et al. 2007; Mizumori et al. 2009). One of the cornerstones of the hypothesis is the presence of pH-dependent AP activity measured in situ. Since AP activity had not previously been measured in intact tissues in living animals, we used the fluorogenic AP substrate ELF-97 to measure AP activity in situ in living rats (Akiba et al. 2007). To vary the pHo, we varied perfusate pH or the rate of HCO3– secretion. Perfusion with a pH 2.2 solution, with a > 7 log greater [H+] than the AP pH optimum, only decreased AP activity (60% remaining in situ) contrasting with the absent activity predicted at pH 2.2 when AP activity is measured in vitro. Augmentation of epithelial HCO3– secretion with an I.V. injection of PGE2 increased the rate of HCO3– secretion, presumably due to elevation of pHo (Fig. 2).
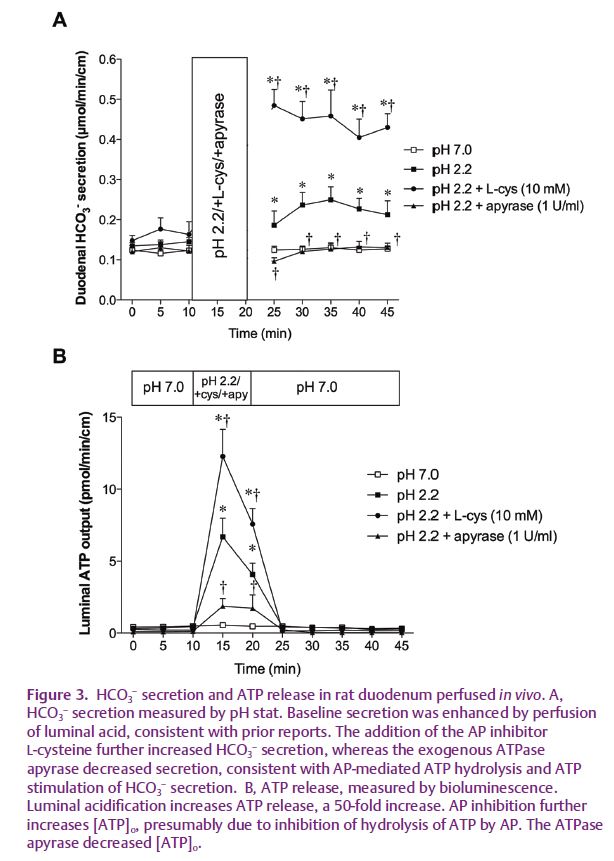
The wide availability of potent and selective purinergic agonists and antagonists considerably simplified the biochemical characterization of duodenal ecto-purinergic signalling. Perfusion of the duodenal lumen with an acidic solution, which increases the rate of HCO3– secretion, releases ATP into the lumen at a concentration ~50-fold higher than during perfusion with a neutral solution (Mizumori et al. 2009). P2Y1 antagonists impair the secretory response to exogenous ATP. Importantly, inhibition of AP activity augments [ATP]o and increases the rate of HCO3– secretion, supporting ATP hydrolysis as an important component of the mechanism (Fig. 3). Inhibition of the CF transmembrane regulator (CFTR) interestingly impairs ATP release, suggesting a possible mechanism whereby CFTR dysfunction impairs HCO3– secretion. Since an alkaline microclimate overlying the duodenal brush border may protect the underlying mucosa from injury due to gastric acid entering the duodenum, we measured injury of the epithelial cells with the normally excluded DNA-binding dye propidium iodide. A P2Y1 antagonist markedly increased cellular damage during acid perfusion (Mizumori et al. 2009), supporting a protective function of the pHo regulatory system.
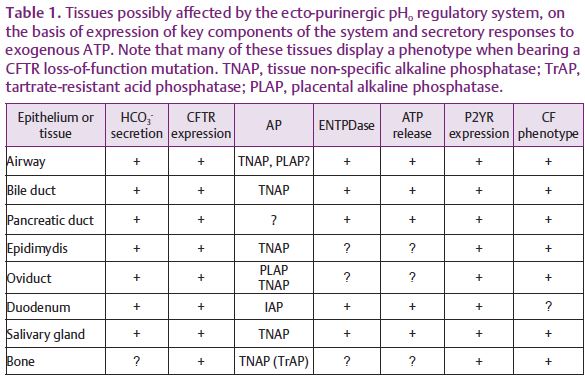
The literature abounds with reports of tissues that are likely candidates for ecto-purinergic pHo regulation due to expression of several of the required components such as surface P2Y receptors and ecto-ATP-hydrolysing enzymes expression combined with the presence of ecto-nucleotide-provoked HCO3– secretion. Interestingly, many of these tissues also express the CFTR as part of the HCO3– secretory mechanism and are part of the phenotype of CFTR loss of function (Table 1). Thus, it is possible that similar ecto-purinergic regulatory systems control pHo in other tissues, where such regulation is important for disparate processes such as sperm capacitation, ciliary beat frequency and bone formation (Kaunitz & Yamaguchi, 2008). We hope that insights derived from further testing of this hypothesis will identify new targets for the treatment of diseases such as CF and other disorders affecting epithelial anion secretion.
References
Akiba Y, Mizumori M, Guth PH, Engel E & Kaunitz JD (2007). Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293, G1223–G1233.
Burnstock G (2007). Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87, 659–797.
Kaunitz JD & Yamaguchi DT (2008). TNAP, TrAP, ecto-purinergic signalling, and bone remodeling. J Cell Biochem 105, 655–662.
Leipziger J (2003). Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284, F419–F432.
Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD & Akiba Y (2009). Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol 587, 3651–3663. http://jp.physoc.org/content/587/14/3651.long
