Physiology News Magazine
Is brain carbohydrate consumption driven by adrenaline?
Many people use exercise to switch the brain off following a stressful day. While low intensity exercise can be performed almost automatically, strenuous exercise requires intense brain activation to keep the muscles working. Brain activation is accompanied by increased carbohydrate uptake and an increase in plasma adrenaline, rather than in noradrenaline, appears to be important for this uptake, suggesting that cerebral glycolysis is driven by a β2-adrenergic mechanism
Features
Is brain carbohydrate consumption driven by adrenaline?
Many people use exercise to switch the brain off following a stressful day. While low intensity exercise can be performed almost automatically, strenuous exercise requires intense brain activation to keep the muscles working. Brain activation is accompanied by increased carbohydrate uptake and an increase in plasma adrenaline, rather than in noradrenaline, appears to be important for this uptake, suggesting that cerebral glycolysis is driven by a β2-adrenergic mechanism
Features
Thomas Seifert
Department of Anaesthesia, The Copenhagen Muscle Research Centre, Faculty of Health Sciences, University of Copenhagen, Denmark
https://doi.org/10.36866/pn.75.20

Cerebral metabolism at rest
At rest, cerebral energy metabolism is covered almost exclusively by oxidation of glucose since the molar ratio between the brain’s oxygen (O2) uptake to that of glucose is ~6. However, glucose is not the only substrate that supports brain metabolism. Lactate is recognized as an energy substrate for neurons and, therefore, the total amount of carbohydrates taken up by the brain relative to that of O2 is considered in the O2–carbohydrate index: OCI [O2/(glucose + ½ lactate)]. A value of ~5.7 is often reported at rest although OCI may be as low as ~4 (Seifert et al. 2009) or above 6.
Cerebral metabolism during exercise
During light exercise OCI is maintained near its resting value, but during intense to maximal exercise, OCI decreases to the lowest reported value of 1.7 during maximal ergometer rowing with an arterial lactate concentration of ~20 mM. The mechanism responsible for the decrease in OCI is not yet established (Dalsgaard, 2003), but OCI decreases independently of O2 availability. Even though cerebral lactate uptake increases in proportion to the arterial lactate concentration, OCI also decreases in the absense of a significant increase in arterial lactate concentration (<2 mM) during prolonged exercise. Also, the uptake of free fatty acids, glycerol, glutamine, alanine and pyruvate cannot explain the decrease in OCI during cerebral activation. Rather, cerebral glycogen metabolism may explain the decrease in OCI since the glycogen deposit in the brain (~10 μmol g–1) is of the same order of magnitude as the surplus uptake of carbohydrate during intense exercise (Dalsgaard, 2006). During cerebral activation the surplus uptake of carbohydrate could replenish the cerebral glycogen deposit.
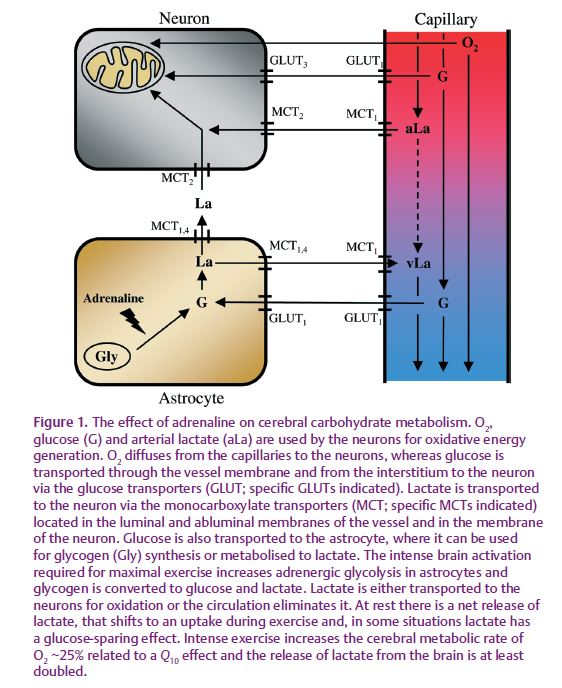
In order to accommodate the increased energy demand during cerebral activation, additional glucose and lactate are taken up by the brain to support oxidation in neurons supplemented by glycolysis in astrocytes that provide lactate to neurons for oxidation. Evidence of increased cerebral glycolysis during brain activation is obtained using 13C-labelled lactate to demonstrate a 2-fold increased lactate uptake at an arterial concentration of ~4 mM, whereas the release is unaffected. However, during exercise with an arterial lactate concentration of ~7 mM, the cerebral lactate uptake increases ~6-fold and the release increases 2-fold (Fig. 1). Since virtually all lactate taken up by the brain is metabolized at rest (~100%) as well as during exercise (~87 %), the increased lactate release from the brain during intense exercise supports the idea that the rate of glycolysis increases with metabolism. Glycolysis may be needed during maximal exercise for which the increase in cerebral O2 consumption becomes so pronounced that both global and regional cerebral oxygenation decrease to a critical level.
Sympathetic influence on cerebral metabolism?
The OCI decreases not only during exercise. In a positron emission tomography (PET)-based evaluation of brain metabolism, OCI decreased in the visual cortex from a resting value of 4.1 to 2.8 in response to intense visual stimulation (Fox et al. 1988). Similarly, a mental task decreases OCI as determined by arterial and internal jugular venous blood sampling (Madsen et al. 1995). Following catheterization, there is some recovery of OCI, e.g. from ~4 to ~5 over an hour (Seifert et al. 2009) suggesting that OCI decreases in response to the associated discomfort (Fig. 2). Such a psychological effect could also explain the low baseline OCI of 4.1 in the study by Fox et al. (1988), maybe reflecting the anxiety provoked by being placed in a scanner. In support, subjects seem to adapt to participating in an experiment with reduced anxiety, as illustrated by a reduced cerebral carbohydrate uptake and, thus, a higher resting OCI when they visit the laboratory for the follow-up (Fig. 2). At that time, the arterial adrenaline concentration is attenuated.
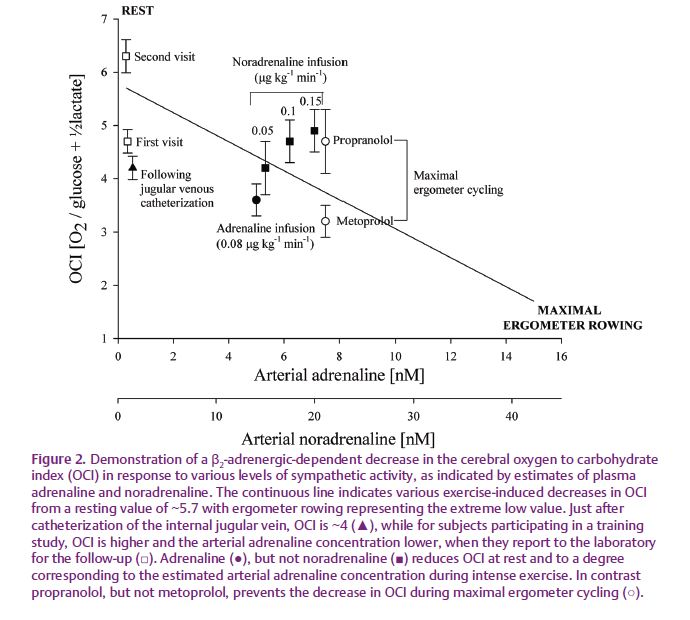
Together these observations indicate that OCI decreases in response to sympathetic stimulation and OCI decreases in response to infusion of adrenaline at a rate establishing an arterial plasma concentration comparable to that elicited during strenuous exercise (70% of maximal oxygen uptake; VO2max) during which OCI decreases (Fig. 1; Seifert et al. 2009). In contrast, infusion of noradrenaline is without an effect on OCI at an arterial concentration comparable to that established during strenuous exercise. When strenuous exercise is carried out with the β1/β2-adrenergic receptor antagonist propranolol, the decrease in OCI is prevented (Larsen et al. 2008), whereas the OCI decreases during exercise with the β1-adrenergic receptor antagonist metoprolol (Dalsgaard, 2006). Thus, circulating adrenaline seems to stimulate cerebral carbohydrate metabolism mediated via a β2-adrenergic receptor mechanism.
How does adrenaline affect cerebral carbohydrate metabolism?
How adrenaline exerts its effect on cerebral metabolism remains speculative. A decrease in OCI supports the idea that glycolysis takes place in astrocytes during intense brain activation since the carbohydrate uptake cannot be accounted for by that of O2 and adrenaline may accelerate cerebral glycogenolysis. The premise for this suggestion is that adrenaline released into the circulation is capable of penetrating the blood–brain barrier. In order to elucidate the role of adrenaline on cerebral carbohydrate uptake, we are awaiting, for example, a tissue culture evaluation of β2-adrenergic influence on glycogen turnover in astrocytes, thereby revealing whether a decrease in OCI is related to breakdown or replenishment of the cerebral glycogen level.
References
Dalsgaard MK (2003). Brain food: The cerebral metabolic response to exercise. Physiol News 53, 29–31.
Dalsgaard MK (2006). Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab 26, 731–750.
Fox PT, Raichle ME, Mintun MA & Dence C (1988). Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464
Larsen TS, Rasmussen P, Overgaard M, Secher NH & Nielsen HB (2008). Non-selective β-adrenergic blockade prevents reduction of the cerebral metabolic ratio during exhaustive exercise in humans. J Physiol 586, 2807–2815.
Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, Wildschiodtz G, Paulson OB & Lassen NA (1995). Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab 15, 485–491
Seifert TS, Brassard P, Jorgensen TB, Hamada AJ, Rasmussen P, Quistorff B, Secher NH & Nielsen HB (2009). Cerebral non-oxidative carbohydrate consumption in humans driven by adrenaline. J Physiol 587, 285–293.