
Physiology News Magazine
Negative consequences of physical inactivity on non-alcoholic fatty liver disease development
Leading a sedentary lifestyle is increasing the risk for development of non-alcoholic fatty liver disease (NAFLD) in westernized countries. Sudden cessation of physical activity appears to upregulate many processes known to initiate hepatic steatosis development
Features
Negative consequences of physical inactivity on non-alcoholic fatty liver disease development
Leading a sedentary lifestyle is increasing the risk for development of non-alcoholic fatty liver disease (NAFLD) in westernized countries. Sudden cessation of physical activity appears to upregulate many processes known to initiate hepatic steatosis development
Features
R Scott Rector (1) & Jamal A Ibdah (1,2,3)
1: Division of Gastroenterology and Hepatology, 2: Harry S. Truman Memorial Veterans Medical Center, and 3: Department of Medical Pharmacology and Physiology, University of Missouri,Columbia, MO 65212, USA.
https://doi.org/10.36866/pn.74.31

Sedentary lifestyle and poor dietary choices are increasing the risk for developing the metabolic syndrome and non-alcoholic fatty liver disease (NAFLD) in westernized societies. NAFLD encompasses a histological spectrum ranging from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis. It is estimated that ~30% of the US adult population has excessive fat accumulation in liver, reaching levels as high as 75–100% in obese and morbidly obese individuals. NAFLD is considered the hepatic manifestation of the metabolic syndrome and is strongly associated with reductions in insulin sensitivity, increased rates of gluconeogenesis, impaired insulin response to suppress gluconeogenesis, and impaired fatty acid oxidation (reviewed by Rector et al. 2008c). Contributing to hepatic steatosis is: (1) excess dietary fat packaged as triglycerides (TG) in chylomicrons, (2) increased free fatty acid (FFA) flux from adipose tissue lipolysis, (3) increased de novo lipogenesis, (4) diminished exportation of TG into very low density lipoproteins, and (5) reduced hepatic fatty acid oxidation.
The present ‘gold standard’ management of NAFLD and NASH includes lifestyle modification targeted at increasing physical activity and reducing energy intake. Weight loss by energy restriction alone or in combination with exercise training has been shown to reduce hepatic fat content. In addition, exercise training in the absence of weight loss also improves hepatic insulin sensitivity by reducing endogenous glucose output (Shojaee-Moradie et al. 2007). Conversely, cross-sectional studies in humans have shown that reduced habitual physical activity (Perseghin et al. 2007) is associated with NAFLD. In addition, less physically active individuals exhibit higher rates of hepatic FFA uptake compared with more active individuals (Hannukainen et al. 2007). More importantly, while the lack of regular exercise or physical inactivity is an ‘actual’ cause of death (Mokdad et al. 2004), the detrimental hepatic alterations that ensue following daily exercise cessation and undertaking a sedentary lifestyle are largely unknown.
We have utilized a commonly studied animal model of obesity and type 2 diabetes, the Otsuka Long–Evans Tokushima Fatty (OLETF) rat, to begin to address these questions. OLETF rats are hyperphagic, become obese and develop insulin resistance, type 2 diabetes, the metabolic syndrome, and NAFLD. However, when OLETF rats are given access to voluntary running wheel and allowed to exercise daily, body weight is suppressed (Rector et al. 2008a), whole-body insulin sensitivity is enhanced, and the development of type 2 diabetes is prevented (Shima et al. 1993). In addition, we have recently expanded these observations and reported that daily exercise also attenuates the development of hepatic steatosis in the OLETF rat in part by increasing hepatic fatty acid oxidation and reducing key de novo lipogenesis proteins (Rector et al. 2008a,b).
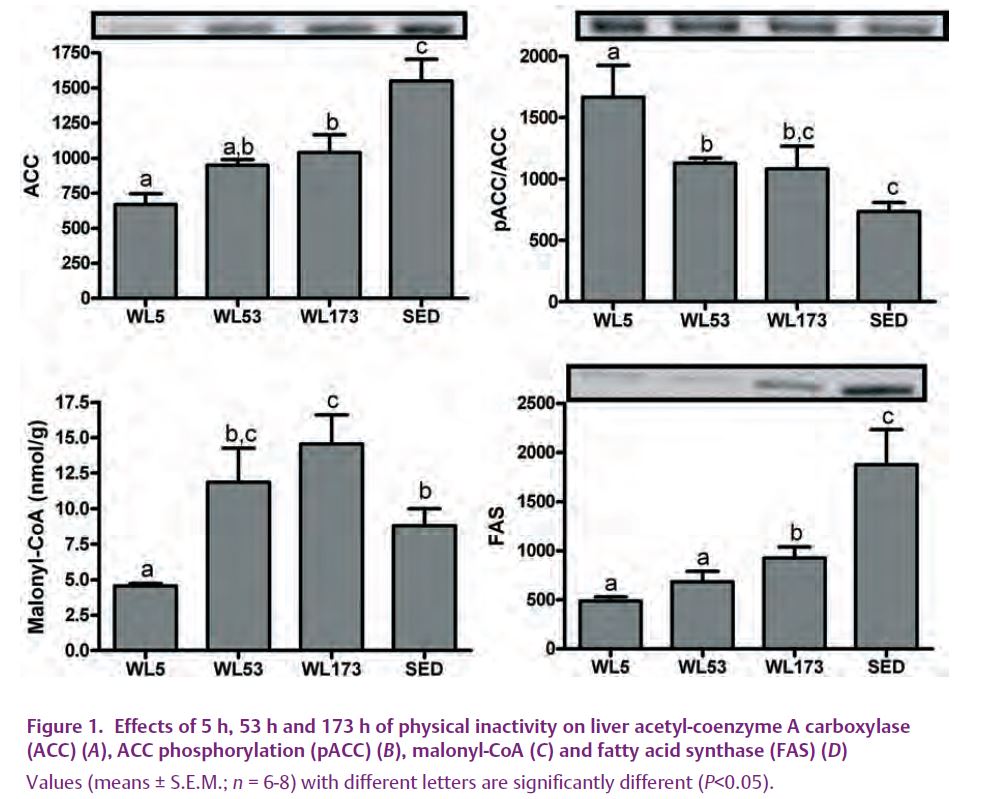
While mounting evidence suggests a negative impact of physical inactivity on the growing problem of NAFLD, little information exists to explain the reason for this occurrence. In order to gain novel mechanistic insight into initial detrimental effects of daily exercise cessation on hepatic lipid metabolism, OLETF rats that had been given access to voluntary running wheels had the wheels locked (wheel lock; WL) for 5 hours (WL5), 53 hours (WL53), or 173 hours (WL173) . To our knowledge, we are the first to report that the sudden cessation of daily exercise in a hyperphagic/obese model activates a subgroup of precursors and processes known to initiate hepatic steatosis (Rector et al. 2008a,b). In spite of no significant change in many peripheral factors previously associated with hepatic steatosis (body weight, fat pad mass, food intake, serum insulin), we found a rapid decline in complete fatty acid oxidation in liver and hepatic mitochondrial enzymes in the days after the cessation of daily exercise. In addition, cessation of daily exercise quickly increased the hepatic protein contents of fatty acid synthase (FAS) and acetyl-coenzyme A carboxylase (ACC), reduced ACC phosphorylation status (resulting in increased activation), and dramatically increased hepatic malonyl-CoA concentrations (Fig. 1), all integral steps in hepatic fatty acid synthesis. Despite the observed increase in malonyl-CoA (known to be associated with increased lipid deposition and reduced fatty acid oxidation) seen with exercise cessation, significant hepatic TG accumulation was not apparent with 173 hours of exercise cessation (Fig. 2) (Rector et al. 2008a). However, it is unlikely that the beneficial effects of daily physical activity on hepatic TG accumulation will persist indefinitely, particularly in a hyperphagic/obese model.
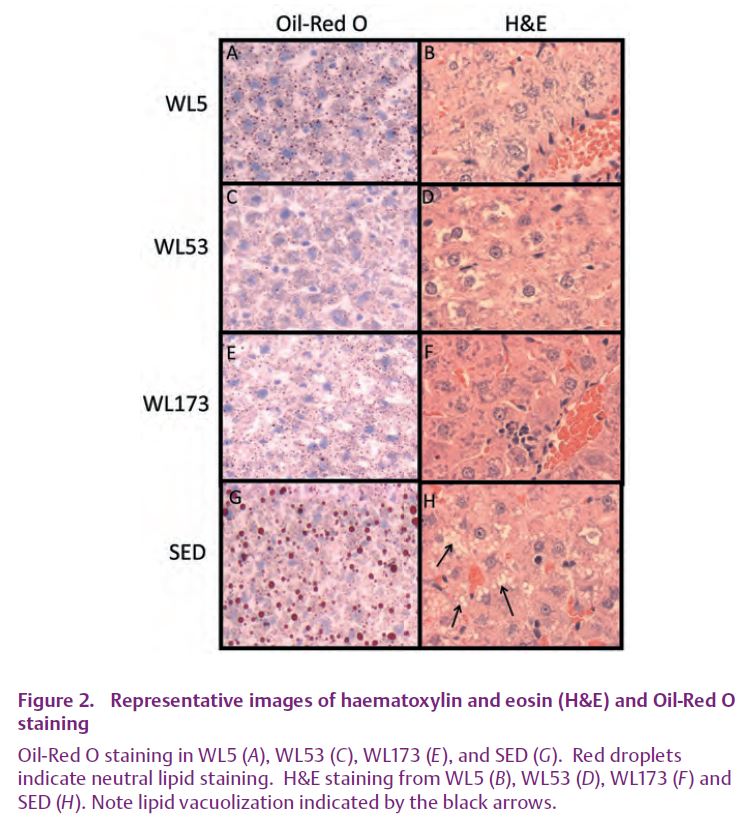
These data strongly suggest that a sudden transition to a sedentary lifestyle increases susceptibility to NAFLD, and probably have important clinical significance as the OLETF rat model might be assimilated to hyperphagic/obese humans who continually stop and start exercise programs. Many populations have adopted increasingly intermittent physically inactive lifestyles at the same time that NAFLD is reaching epidemic proportions in the United States and westernized societies. Using exercise cessation as a tool may provide an opportunity to understand the time course of molecular/biochemical events that lead to excessive hepatic fat accumulation, and thus future time course studies are warranted.
References
Hannukainen JC, Nuutila P, Borra R, Kaprio J, Kujala UM, Janatuinen T, Heinonen OJ, Kapanen J, Viljanen T, Haaparanta M, Rönnemaa T, Parkkola R, Knuuti J & Kalliokoski KK (2007). Increased physical activity decreases hepatic free fatty acid uptake: a study in human monozygotic twins. J Physiol 578, 347–358.
Mokdad AH, Marks JS, Stroup DF & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA 291, 1238–1245.
Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A & Luzi L (2007). Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 30, 683–688.
Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW & Ibdah JA (2008a). Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586, 4241–4249.
Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW & Ibdah JA (2008b). Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294, G619–G626.
Rector RS, Thyfault JP, Wei Y & Ibdah JA (2008c). Non-alcoholic fatty liver disease and the metabolic syndrome: An update. World J Gastroenterol 14, 185–192.
Shima K, Shi K, Sano T, Iwami T, Mizuno A & Noma Y (1993). Is exercise training effective in preventing diabetes mellitus in the Otsuka-Long-Evans-Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Metabolism 42, 971–977.
Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, Whyte M, Lovell D, Bowes SB, Gibney J, Jones RH & Umpleby AM (2007). Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 50, 404–413.
