
Physiology News Magazine
How many sensors in the bladder?
Sensory neurons projecting from the urinary bladder (bladder afferents) play a key role in neural circuits underlying both urine storage and normal micturition. They are responsible for all sensations from the bladder such as fullness, the urge to micturate, discomfort and pain. Afferents from different regions of the bladder probably have characteristic receptive fields, response properties and conduction velocities. Indeed, recent experiments in vitro have identified several distinct functional classes of bladder afferents. How then can we relate the functions of various types of afferents in normal and pathological states of the urinary bladder?
Features
How many sensors in the bladder?
Sensory neurons projecting from the urinary bladder (bladder afferents) play a key role in neural circuits underlying both urine storage and normal micturition. They are responsible for all sensations from the bladder such as fullness, the urge to micturate, discomfort and pain. Afferents from different regions of the bladder probably have characteristic receptive fields, response properties and conduction velocities. Indeed, recent experiments in vitro have identified several distinct functional classes of bladder afferents. How then can we relate the functions of various types of afferents in normal and pathological states of the urinary bladder?
Features
Vladimir Zagorodnyuk, Ian Gibbins, Marcello Costa, Simon Brookes & Sarah Gregory
Departments of Human Physiology and Anatomy & Histology, Centre for Neuroscience, Flinders University, Adelaide, Australia
https://doi.org/10.36866/pn.73.18

Based on their conduction velocities, bladder afferents comprise two main groups: faster conducting, thinly myelinated Aδ-fibres; slower conducting unmyelinated C-fibres. In cat bladder, most Aδ-fibres are distension-sensitive, while C fibres are not (Janig, 2006). In rats, however, about 50% of C-fibres respond with low threshold to bladder distension (Shea et al. 2000). Overall, most bladder afferents seem to be polymodal, since they can respond to both mechanical and chemical stimuli (Daly et al. 2007; Rong et al. 2002; Xu & Gebhart, 2008; Zagorodnyuk et al. 2007). Nevertheless, there is considerable diversity in the range of stimuli that activate different classes of bladder afferents.
Mechanoreceptors
Most studies of bladder afferents in vivo have identified mechano-receptors that fire in proportion to intravesical pressure, reflecting the combination of passive distension and active contraction of the bladder wall. Thus, they behave as bladder wall tension receptors. Experiments in vitro have revealed three distinct classes of stretch-sensitive afferents that behave as tension receptors: low threshold mechanoreceptors in the muscle layer; mechanoreceptors at the interface between muscle and mucosa (muscle-mucosal mechanoreceptors); and high threshold mechanoreceptors in the muscle layer (Daly et al. 2007; Rong et al. 2002; Xu & Gebhart, 2008; Zagorodnyuk et al. 2007). There also may be ‘volume’ receptors, which sense bladder distension irrespective of pressure (Morrison, 1999).
It is widely believed that low threshold mechanoreceptors are involved in non-painful sensation from the bladder and contribute to reflex regulation of bladder contractility. In contrast, high threshold afferents mediate discomfort and painful sensations from the bladder (de Groat, 2006; Janig 2006). However, this division has numerous pitfalls. Firstly, low threshold, wide dynamic range mechanoreceptors encode a large range of mechanical stimuli (distension and contraction) extending into the noxious range. Secondly, only some high threshold mechanoreceptors respond to distension (Janig, 2006). To complicate matters further, some low threshold mechanoreceptors express capsaicin-sensitive TRPV1 receptors (Daly et al. 2007) that normally are associated with functional nociceptors.
Chemoreceptors
It is still unclear whether pure chemoreceptors exist in the bladder or if chemoreception is mediated by polymodal stretch-insensitive mechanoreceptors. Some bladder afferents are excited in vivo only by distension with isotonic KCl, but not NaCl, and these afferents have been considered to be chemoreceptors (Moss et al. 1977). Stretch-insensitive afferents activated by chemical stimuli (capsaicin, hypertonic solution, α,β-methylene ATP) in vitro probably correspond to chemoreceptors described in vivo. However, the same fibres also respond vigorously to gentle mucosal stroking (mucosal high-responding mechanoreceptors), indicating that they are actually polymodal afferents (Zagorodnyuk et al. 2007). Patients with interstitial cystitis have enhanced sensitivity to intravesicular instillation of hyper-tonic KCl solutions, suggesting that some of their symptoms may be due to increased firing of afferents with these properties.
Nociceptors
A widely-used classification identifies two broad categories of small diameter, slowly-conducting nociceptive neurons:
- those expressing neuropeptides [typically, substance P (SP) and calcitonin gene-related peptide (CGRP)] plus TRPV1 and TrkA receptors;
- those that do not, but which are labelled by the isolectin B4 (IB4), and depend on glial-derived neuronotrophic factor (GDNF) for postnatal survival.
However, this is almost certainly a misleading simplification.
Potentially nociceptive C-fibre neurons comprise a functionally diverse array characterised by differential expression of various TRP and ASIC channels responding to temperature and pH, TTX-resistant Na+ channels (Nav1.8 and 1.9) and a range of K+ channels (Fang et al. 2006). How these functional characterisations correlate with the broad neurochemical categories is still largely unresolved. For example, intravesical instillation of capsaicin, which opens TRPV1 channels, produces burning pain in humans or pain-related behaviour in animals. However, in guinea pig bladder, capsaicin activates at least two different classes of mechanoceptive afferents: high threshold stretch-insensitive mechanoreceptors and the mucosal high-responding mechanoreceptors which are presumably activated in damaged or inflamed urothelium (Zagorodnyuk et al. 2007).
As with other nociceptors, both low and high threshold mechanoreceptors in the bladder show sensitisation by inflammatory mediators, such as cytokines, α,β-methylene ATP or as a result of cystitis (Rong et al. 2002; Roppolo et al. 2005; Xu & Gebhart, 2008). Thus, noxious stimuli in the urinary bladder probably are detected by thin myelinated Aδ- and unmyelinated C-fibres alike, both of which include low and high threshold mechano-receptors.
‘Silent’ afferents
Up to about 30% of afferent to the bladder apparently do not respond to any level of distension and have been called ‘silent afferents’ . However, acute inflammation induces some previously ‘silent afferents’ to become spontaneously active and develop a degree of mechanosensitivity. Thus, they may contribute to nociception from the inflamed bladder (Janig, 2006). A subset of them may represent stretch-insensitive mechano-receptors with endings in the mucosa (mucosal low responding mechanoreceptors). So-called ‘cold receptors’ that express TRPM8 (a TRP channel responsible for detecting cold stimuli) probably represent another class of ‘silent nociceptors’ in the bladder, since their density is increased in the suburothelium of overactive and painful bladders (Mukerji et al. 2006).
Linking structure and function
It is commonly believed that all visceral afferents have unspecialised bare endings (Janig, 2006). In rats, only 50–60% of spinal sensory neurones projecting to the bladder are labelled with neuropeptides such as CGRP. So far we do not have a reliable neurochemical marker for visceral afferent endings that do not express neuropeptides. Consequent-ly, nearly half the bladder afferents never have been specifically visualised. Now, however, we can reveal non-peptide bladder afferents by anterogradely labelling functionally-characterised fibres with biotinamide (Fig. 1). Following this procedure, several morphological types of bladder afferent endings can be seen: ‘antenna-like endings’ in the muscle layers, ‘grape-like-endings’ in the lamina propria; and free varicose endings in the lamina propria (Fig. 1C, D & F). Most probably, ‘antenna-like endings’ correspond to muscle mechanoreceptors, while ‘grape-like endings’ are muscle-mucosal mechanoreceptors. Free nerve endings containing CGRP and SP in the lamina propria (Fig. 1E & F) probably represent capsaicin-sensitive mucosal mechano-receptors, since removal of the urothelium both abolished their response to light von Frey hair stroking (Zagorodnyuk et al. 2007) and produced significant damage of SP- and CGRP-positive nerve endings in the lamina propria.
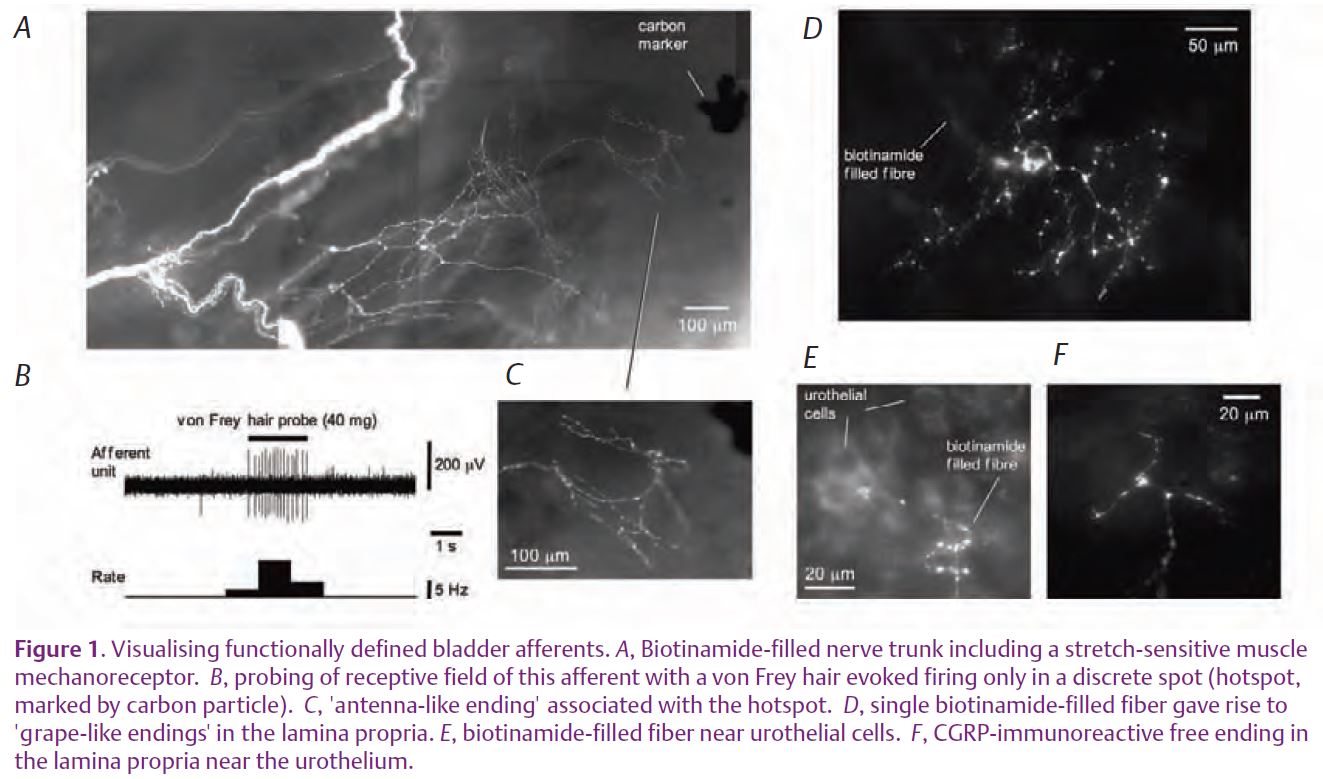
Clearly, there is great complexity in the sensory innervation of the bladder. There must be around 10 distinct functional classes, each of which has a precise role in signalling the mechanical and chemical environment of the bladder. An immediate challenge is to characterise each of them fully according to morphological, functional, pharmacological and immunohistochemical criteria. Only then, will we be able to determine which populations of afferents are most important clinically, and most amenable for pharmacological manipulation in the treatment of bladder disorders.
Acknowledgements
This study was funded by National Heath and Medical Research Council of Australia grant no. 375123.
References
Daly D, Rong W, Chess-Williams R, Chaple C & Grundy D (2007). Bladder afferent sensitivity in wildtype and TRPV1 knockout mice. J Physiol 583, 663–674.
de Groat WC (2006). Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147, S25–S40.
Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K & Lawson SN (2006). Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes c-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci 26, 7281–7292.
Janig W (2006). The integrative action of the autonomic nervous system. Cambridge University Press, Cambridge, UK.
Morrison JF (1999). The activation of bladder wall afferent nerves. Exp Physiol 84, 131–136.
Moss NG, Harrington WW & Tucker MS (1997). Pressure, volume and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol 272, R695–R703.
Mukerji G, Yiangou Y, Corcoran S, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK & Anand P (2006). Cool and mentol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urology 6, 6. doi:10.1186/147I-2490-6-6.
Rong W, Spyer KM & Burnstock G (2002). Activation and sensitisation of low and high threshold afferent fibers mediated by P2X receptors in the mouse urinary bladder. J Physiol 541, 591–600.
Roppolo JR, Tai C, Booth AM, Buffington CAT, de Groat WC & Birder LA (2005). Bladder Aδ afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol 173, 1011–1015.
Shea VK, Cai R, Crepps B, Mason JL & Perl ER (2000). Sensory fibers of the pelvic nerve innervating the rat’s urinary bladder. J Neurophysiol 84,1924–1933.
Xu L & Gebhart GF (2008). Characterization of mouse lumbar splanchnic and pelvic urinary bladder mechanosensory afferents. J Neurophysiol 99, 244–253.
Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJH & Gregory SJ (2007). Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol 585, 147–163.
