
Physiology News Magazine
Force-frequency relation and myofilament Ca2+ sensitivity
In the failing heart alterations in Ca2+ sensitivity of the myofilaments contribute to the decrease in pump function when heart rate increases, for instance during exercise. An altered balance between kinase and phosphatase activity, which influences phosphorylation and thereby function of the myofilament protein troponin I appears to be responsible
Features
Force-frequency relation and myofilament Ca2+ sensitivity
In the failing heart alterations in Ca2+ sensitivity of the myofilaments contribute to the decrease in pump function when heart rate increases, for instance during exercise. An altered balance between kinase and phosphatase activity, which influences phosphorylation and thereby function of the myofilament protein troponin I appears to be responsible
Features
Regis R Lamberts, Jolanda van der Velden, & Ger J M Stienen
VU University Medical Center, Amsterdam, The Netherlands
https://doi.org/10.36866/pn.71.39

In a number of mammalian species, including man, an increase in heart rate results in an enhanced pump function of the heart. The positive pressure-frequency relationship was first described by Bowditch in the isolated frog heart (Bowditch, 1871) and is also known as the Treppe or staircase phenomenon. In the failing heart, where pump function is impaired, a blunted or negative pressure-frequency or force-frequency relation is observed (Gwathmey et al. 1990). Fig. 1 illustrates a positive and a negative Treppe in isolated healthy and failing rat cardiac muscles, respectively. The negative force-frequency relation will markedly impede the exercise capacity of the heart, one of the defining symptoms of patients with heart failure.

The positive force-frequency relation has been attributed to an increased influx of Ca2+ ions per unit of time in myocardial cells. This results in an increase in Ca2+ content of the sarcoplasmic reticulum and thereby in an increase in the amplitude of the intracellular Ca2+ transient during contraction. During heart failure the intracellular Ca2+ content is reduced, in a frequency-dependent manner by alterations in the Ca2+- and Na+-ion homeostasis.
However, not only the amount of Ca2+, but also the sensitivity of the contractile myofilaments for Ca2+ determines the magnitude of force developed by the cardiomyocyte. Myofilament Ca2+ sensitivity is modulated by the phosphorylation levels of the regulatory thin and thick myofilament proteins, troponin I (TnI) and myosin light chain 2 (MLC2)(e.g. van der Velden et al. 2003). Beta-adrenergic stimulation during exercise accelerates heart rate, but also activates the classical second messenger cAMP dependent pathway, resulting in activation of protein kinase A (PKA). PKA phosphorylates, amongst others, serines 23 and 24 of the inhibitory subunit of the troponin complex TnI. This addition of phosphate groups, i.e. negative charge, causes a reduction in Ca2+ sensitivity of the myofilaments and thus a reduction in peak force during contraction (systole). Phosphorylation of TnI is also implicated in an acceleration of cellular relaxation and thereby promotes filling of the ventricles with blood during the resting (diastolic) phase of the cycle. Intriguingly, there have been some reports suggesting that an increase in pacing frequency is accompanied by an increase in myofilament Ca2+-sensitivity.
During heart failure Ca2+ sensitivity was found to be increased in human (van der Velden et al 2003) and rat myocardium (Lamberts et al. 2007), though this is not an universal finding. The increased Ca2+ sensitivity in heart failure has been attributed to a reduced PKA-mediated phosphorylation of TnI as a result of downregulation and/or desensitisation of the β-adrenergic receptors. This could modulate a putative effect of contractile protein phosphorylation on Ca2+ sensitivity of the myofilaments.
Therefore we addressed the following question: Do alterations in Ca2+ sensitivity, by a change in phosphorylation of the myofilament contractile proteins, contribute to the force-frequency relation in healthy hearts and is this also the case in failing hearts?
The first clues were obtained from force and intracellular Ca2+ transient measurements in rat cardiac trabeculae. These results revealed a parallel increase in force and amplitude of the Ca2+ transient in healthy control rats, but a decline in force in trabeculae from failing hearts without a change in the amplitude of the Ca2+ transient. This suggested that changes in myofilament Ca2+ sensitivity have occurred.
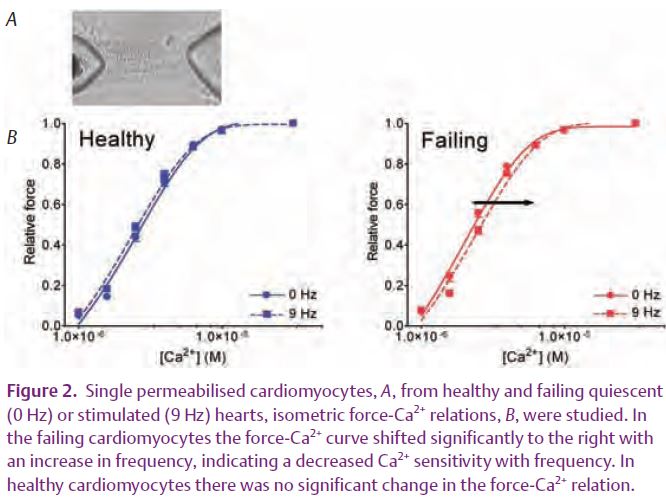
Subsequently, we studied myofilament Ca2+ sensitivity in permeabilised cardiomyocytes, isolated from quiescent and electrically paced hearts (9 Hz) perfused in a Langendorff set-up (Fig. 2). In the control cells, the force-Ca2+ relations were practically identical, indicating that the Ca2+ sensitivity of the contractile apparatus remained unaltered. In cardiomyocytes from failing rat hearts, the force-Ca2+ relation shifted to the right, indicating a decrease in Ca2+ sensitivity with pacing frequency. Determination of the phosphorylation levels of TnI and MLC2 by one and two dimensional gel electrophoresis revealed that the phosphorylation levels of both proteins remained constant in healthy hearts but that both increased with pacing frequency in failing hearts (Lamberts et al. 2007). Thus, in the healthy heart, the increase in Ca2+ influx per unit of time is primarily responsible for the positive force-frequency relation. However, in the failing heart, frequency-dependent Ca2+ desensitisation of the myofilaments together with alterations in Ca2+ homeostasis result in a negative force-frequency relation. This frequency-dependent Ca2+ desensitisation can be attributed to a frequency-dependent increase in phosphorylation of the actin-myofilament protein TnI, because MLC2 phosphorylation would have the opposite effect.
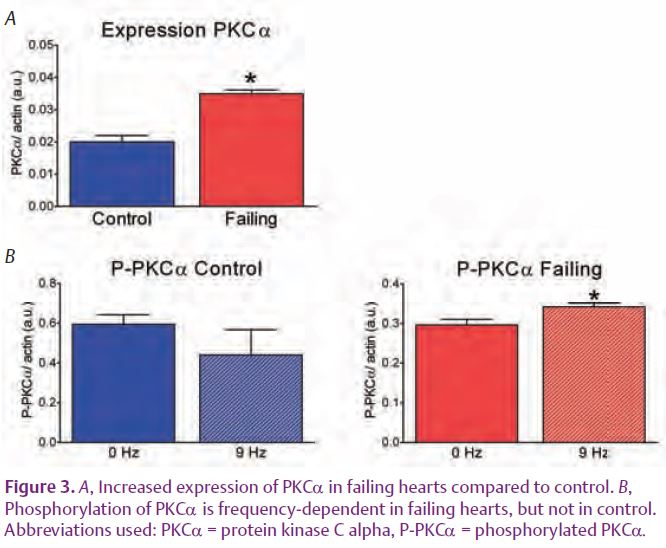
The next question is: how does stimulation frequency influence TnI phosphorylation? The answer to this question should reside in an altered balance of kinase or phosphatase activity. In particular, the Ca2+-dependent kinases or phosphatases might be involved because the time-averaged intracellular Ca2+ concentration still increases with pacing frequency in both groups. Hence the main candidates would be the classical Ca2+-dependent isoforms of protein kinase C (PKC) and the Ca2+-dependent phosphatase calcineurin (PP2B). PKCα and PP2B are up regulated in heart failure (Braz et al. 2004) and thus their contributions may be more conspicuous in failing than in healthy hearts. In agreement with these findings, we recently showed an increased protein expression of one of the Ca2+ dependent isoform, PKCα, in failing rat hearts. In addition, a modest but significant increase in the phosphorylation level of PKCa with frequency in the failing hearts was found (Fig. 3) (Lamberts et al. 2007).
In summary, in the failing heart alterations in Ca2+ sensitivity of the myofilaments contribute to its negative force-frequency relation. The frequency-dependent changes in phosphorylation levels of PKC-phosphorylatable sites of TnI originate from an altered balance in kinase and phosphatase activity, once more indicating the importance of kinase/phosphatase activity in regulation of cardiac function.
References
Bowditch HP (1871). Über die Eigenthümlichkeiten der Reizbarkeit, welche die Musklefasern des Herzens zeigen. Berichte über die verhandlungen der Könglich Sächsischen Gesellschaft der Wissenschaften Zu Leipzig Mathematisch-Physische Klasse 23, 652–689.
Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG & Molkentin JD (2004). PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10, 248–254.
Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM & Morgan JP (1990). Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest 85, 1599–1613.
Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, de Tombe PP, van der Velden J & Stienen GJM (2007). Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol 582, 695–709.
van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K Stienen GJM (2003). Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 57, 37–47.
