
Physiology News Magazine
Cold facts about AMPK in fat
Features
Cold facts about AMPK in fat
Features
Kurt W Saupe & Jacob D Mulligan
Departments of Medicine and Physiology, University of Wisconsin, Madison, WI, USA
https://doi.org/10.36866/pn.70.14

Adenosine monophosphate activated protein kinase (AMPK) has been called the ‘metabolic master switch’, and ‘cellular fuel gauge’ because of its ability to sense intracellular energetic stress in the form of a decreased ATP/AMP ratio and respond by increasing ATP synthesis and decreasing ATP consumption (Hardie & Carling, 1997). Here recent findings that AMPK is highly abundant in brown adipose tissue and regulated in novel ways in both brown and white adipose tissue are discussed. These findings suggest possible novel roles for AMPK in regulation of body weight, body temperature, and pathogenesis during hyper-adrenergic states.
AMPK activity in brown adipose tissue
During the last 15 years, studies conducted largely in liver and muscle have shown that activation of AMPK stimulates ATP producing reactions such as free fatty acid oxidation, while inhibiting ATP consuming processes. This has led to the perspective that high AMPK activity is associated with a state of cellular energy conservation. Brown adipose tissue is in some ways the antithesis of an energy conserving tissue since its uncoupled metabolism converts reducing equivalents into heat instead of useful chemical energy (ATP), in a process termed non-shivering thermogenesis. Thus it was surprising to find that α1 AMPK expression and activity in BAT is the highest yet for any reported tissue, being 3-fold higher than in liver (Mulligan et al. 2007). This AMPK activity is entirely attributable to the α1 isoform, occurs in multiple species (Fig. 1) and appears to be secondary to high levels of expression of the protein.
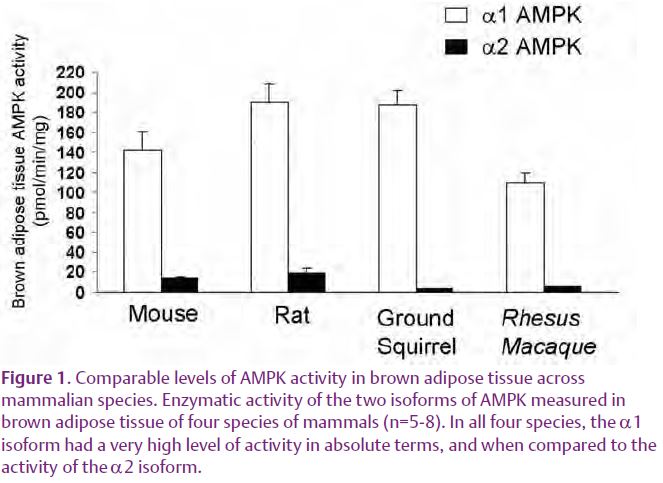
While currently we can only speculate as to the physiological role this high level of AMPK activity plays in brown adipose tissue, it is accompanied by extremely high levels of its downstream target, acetyl-coA carboxylase (ACC), the rate-limiting step in fatty acid synthesis (ACC1) and inhibition of fatty acid oxidation (ACC2). Both ACC isoforms are heavily phosphorylated (inhibited) in brown adipose tissue, presumably by AMPK. Such a massive pool of ACC would be ideal for the simultaneous lipogenesis and β-oxidation that takes place on a large-scale during non-shivering thermogenesis. Upon induction of non-shivering thermogenesis, selective dephosphorylation of ACC1 by an unknown effector might rapidly release the inhibition of fatty acid synthesis, while ACC2 remains inhibited by AMPK, allowing β-oxidation. It is also tempting to speculate that a high AMPK activity is necessary to maintain the high mitochondrial density present in brown adipocytes. This speculation is based on reports that AMPK activates PGC-1α, a transcription factor central to mitochondrial biogenesis (Jager et al. 2007).
AMPK activity in brown adipose tissue during acute and chronic cold exposure
A main function of brown adipose tissue is generation of heat via non-shivering thermogenesis during times of environmental cold exposure. During acute cold exposure this response includes a large increase in oxidation of free fatty acids. Given the established role of AMPK in regulating free fatty acid oxidation in tissues such as liver and muscle, one might anticipate that activation of AMPK would be needed to allow the increase in free fatty acid oxidation during the first hours of cold exposure. Instead, during the first 1–24 hours of exposure to 4° C, AMPK activity does not increase, suggesting that further activation of AMPK above the high basal level is not necessary for the initial induction of non-shivering thermogenesis. It should be noted that in cultured brown adipocytes adrenergic stimulation increases the amount of phosphorylated AMPK within minutes (Hutchinson et al. 2005). However, the meaning of this intriguing finding is unclear since in vivo no increase in AMPK activity occurs during the first hours of cold exposure.
Chronic cold exposure causes brown adipose tissue to remodel in a way that increases its heat generating capacity including tissue hypertrophy, mitochondrial biogenesis and upregulation of UCP1 and other enzymes important for non-shivering thermogenesis. We found that the expression and activity of AMPK increases significantly in brown adipose tissue in response to chronic (7–14 day) cold exposure. Critical insight into the physiological role of AMPK in brown adipose tissue will likely come from determining which of the many cell types within brown adipose tissue contain high levels of AMPK activity that are further upregulated in response to chronic cold exposure. While one might assume that it is the brown adipocytes, this has not yet been established.
Chronic cold-induced upregulation of AMPK also raises the questions of whether the high AMPK activity observed in brown adipose tissue at ‘baseline’ is in part a response to the relative cool temperature at which experimental mice are usually housed (approximately 5° C below their thermoneutral temperature), and whether chronic environmental heat exposure might cause down-regulation of AMPK in brown adipose tissue.
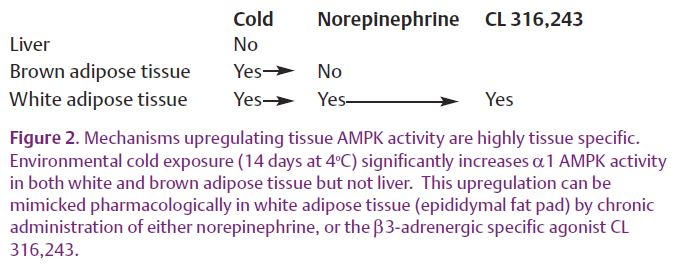
What is the mechanism by which chronic cold exposure increases AMPK activity in brown adipose tissue? Since most, if not all, cold-induced changes in brown adipose tissue biology are mediated by a cold-induced increase in sympathetic nerve activity, we tested whether the effects of cold on AMPK activity could be reproduced by sympathomimetics. However, neither chronic norepinephrine nor the β3-adrenergic agonist CL 316,243 administered to mice for 14 days using implanted micro-osmotic pumps caused an upregulation of brown adipose tissue AMPK activity. This despite the fact that these sympathomimetic drugs caused the expected upregulation of UCP1 levels. This indicates that upregulation of AMPK does not go hand-in-hand with upregulation of UCP1 and other enzymes linked to non-shivering thermogenesis, and suggests the existence of an alternative non-sympathetic mediated pathway for cold-induced gene regulation in brown adipose tissue.
Link between sympathetic nervous system and AMPK in WAT
While white adipose tissue was once regarded largely as an inert storage depot for excess energy, it is now recognized as an organ central to the etiology of diseases such as type 2 diabetes mellitus. A major part of this new understanding comes from the recognition that white adipose tissue is an endocrine organ that depending upon its state of ‘health’ , produces variable amounts of the adipokines that regulate factors such as appetite and insulin sensitivity. We found that in white adipose tissue AMPK activity was robustly upregulated by chronic cold exposure, as it was in brown adipose tissue (Fig. 2). However, in contrast to brown adipose tissue, administration of norepinephrine for 14 days in vivo significantly increased white adipose tissue AMPK activity. To our knowledge, this is the first demonstration of an in vivo link between these two important stress response systems. To identify the adrenergic receptor subtype responsible for this effect, the β3-specific agonist CL 316,243 was administered. It too was sufficient to increase AMPK activity in white adipose tissue, narrowing the search for the relevant cell type to those containing β3-adrenergic receptors (white and brown adipocytes). However, it seems unlikely that the brown adipocytes located within white fat increase AMPK in response to β3 adrenergic stimulation since β3-adrenergic stimulation does not upregulate AMPK in brown adipose tissue.
Significance for human pathophysiology
AMPK is now recognized to play a key role in regulating aspects of whole body energy balance such as appetite (Minokoshi et al. 2004). A role for AMPK in regulating caloric expenditure by brown adipose tissue may be a second way in which AMPK contributes to the regulation of body weight. Given the central role of AMPK in regulating cellular metabolism, its high activity in brown adipose tissue suggests the intriguing possibility that by manipulating AMPK activity one could regulate the number of calories dissipated as heat. ‘Firing up’ brown adipose tissue to treating obesity in humans has gained support from a recent report identifying small, distinct regions of brown adipose tissue in humans –primarily in the area of the collar bone and along the spine (Nedergaard et al. 2007). Future studies will be required to determine what role AMPK in brown adipose tissue plays in regulating body weight and body temperature.
Congestive heart failure, metabolic syndrome and obesity are often hyperadrenergic states that cause desensitization of adrenergic signaling in peripheral tissues. Our data raise the intriguing possibility that desensitization of adrenergic signaling might lead to a loss of AMPK activity in the white adipose tissue of these patients. The idea that loss of AMPK activity can be central to pathology is supported by the successful clinical use of the AMPK activators metformin and thiazolidinediones to treat type 2 diabetes (Musi & Goodyear, 2006). Currently, we are at the very early stage in understanding how AMPK activity is regulated in adipose tissues, and the (patho)physiological meanings of this regulation. As during the last decade, our perspective on AMPK continues to expand, with a new focus on its multiple roles in whole body energy balance.
References
Hardie DG & Carling D (1997). The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem 246, 259–273.
Hutchinson DS, Chernogubova E, Dallner OS, Cannon B & Bengtsson T (2005). Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 48, 2386–2395.
Jager S, Handschin C, St-Pierre J & Spiegelman BM (2007). AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104, 12017–12022.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ & Kahn BB (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574.
Mulligan JD, Gonzalez AA, Stewart AM, Carey HV & Saupe KW (2007). Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol 580, 677–684.
Musi N & Goodyear LJ (2006). Insulin resistance and improvements in signal transduction. Endocrine 29, 73–80.
Nedergaard J, Bengtsson T & Cannon B (2007). Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293, E444–452.
