
Physiology News Magazine
Lack of a role for arterial chemoreceptors in the breakpoint of breath-holding
Oxygen levels in the blood influence breath-hold duration, yet the peripheral chemoreceptors that detect arterial hypoxia appear to have little or no role in determining the breakpoint of breath-holding. Debate about this paradox may have eclipsed another important feature of the breakpoint mechanism
Features
Lack of a role for arterial chemoreceptors in the breakpoint of breath-holding
Oxygen levels in the blood influence breath-hold duration, yet the peripheral chemoreceptors that detect arterial hypoxia appear to have little or no role in determining the breakpoint of breath-holding. Debate about this paradox may have eclipsed another important feature of the breakpoint mechanism
Features
Michael J Parkes
School of Sport & Exercise Sciences, University of Birmingham, Birmingham, UK
https://doi.org/10.36866/pn.70.34

It is a truism that the longer you hold your breath, the lower the partial pressure of oxygen in arterial blood (PaO2) falls and the higher that of carbon dioxide (PaCO2) rises. It is also well established that breath-hold duration can be almost doubled by preoxygenation and almost halved by previously breathing hypoxic gas mixtures (Parkes, 2006). Since aortic arterial chemoreceptors make no demonstrable contribution to breathing in humans (Guz et al. 1966a; Lugliani et al. 1971), the carotid chemoreceptors are the only known means of detecting Pa02 that might influence respiratory control. For this reason there has always been a determination to implicate carotid arterial chemoreceptors in the mechanism explaining the break-point of breath-holding. This is despite the following evidence that they have little or no role!
First, if the carotid chemoreceptors and Pa02 were crucial in controlling the breakpoint, it would be simplest to expect the PaO2 at breakpoint to be constant, i.e. a PaO2 threshold should exist beyond which breath-holding is impossible. PaO2 is normally around 100 mmHg in eupnea. The mean PaO2 at breakpoint is typically 62 ±4 mmHg for breath-holds from maximal lung inflation with air (Lin et al. 1974). This low PaO2 at breakpoint, however, is not constant. A breakpoint still occurs for breath-holds from maximal inflation with 100% O2, even though the PaO2 at breakpoint (Lin et al. 1974) is remarkably higher (553 ±16 mmHg). Conversely, following maximal inflation with hypoxic gas mixtures, the PaO2 at breakpoint is much lower (24-33 mmHg) than usually seen at breakpoint (Ferris et al. 1946).
Secondly, it cannot even be the case that under some of these conditions there is a reinforcing stimulus to carotid chemoreceptors from the PaCO2. The PaCO2 at breakpoint is not constant either (Parkes, 2006). A breakpoint still occurs even if humans breath-hold from low PaCO2 levels (following hyperventilation and in a high oxygen mixture with maximum inflation) and blood gas levels at such breakpoints can be remarkably benign (Cooper et al. 2003; Parkes, 2006).

Thirdly, if the carotid chemo-receptors were crucial in controlling the breakpoint, indefinite breath-holding (until unconsciousness) should be possible following bilateral carotid body resection. Such bilateral destruction of the carotid chemoreceptor afferents was originally performed to attempt to treat asthma and results in failure to increase breathing in 12% hypoxia and to respond to hypercapnia via peripheral chemoreceptors (Davidson et al. 1974; Gross et al. 1976). Yet such indefinite breath-holding is not seen. Fig. 1 shows that their breakpoints persist. There is no difference in mean breath-hold duration vs. intact controls following maximal inflation with 100% O2. Fig.1 nevertheless does show that mean breath-hold duration is 54–65% longer in denervated patients following maximal inflation with either air or hypoxic gas mixtures. This prolongation has never been explained. One interpretation is that it merely represents a difference between the healthy controls and these severe asthmatics, since breath-hold duration was not measured before operation. (Prolonged breath-hold duration was not seen, however, in other asthmatics). Thus carotid chemo-receptors may have no role in the breakpoint. Another explanation is that the absence of chemoreceptor afferents contributes to a lessening of the sensation of discomfort that accompanies breath-holding in air and hypoxia. Thus carotid chemo-receptors may make some little contribution to the duration and hence to the breakpoint. These differences have never been resolved and the experiments may not be repeatable with modern ethical concerns.
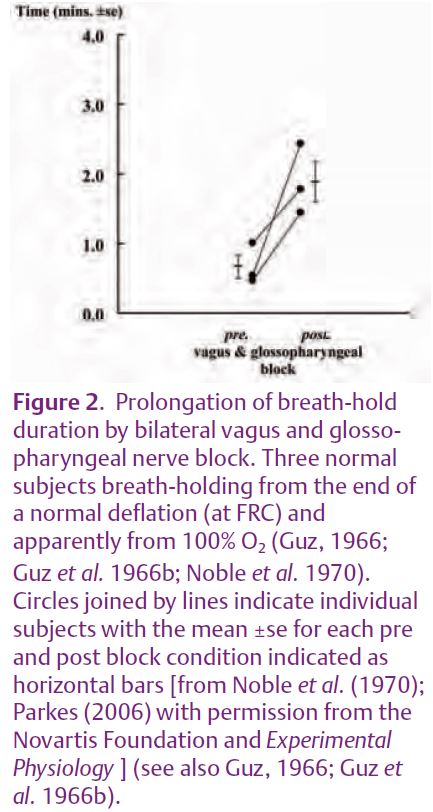
The reason for publicising this debate about carotid chemoreceptors is that it may have eclipsed one further, even older experiment that may be important for our understanding of the breakpoint mechanism. In 1966–1970 Noble et al. (1970) infiltrated local anaesthetic into regions around the glossopharyngeal (IX) and vagus (X) nerves bilaterally in three normal, conscious subjects. Adequate cranial nerve blockade was established by inability to swallow (X & IX), almost complete absence of phonation (X), development of a bovine cough (X), absence of cough to ammonia (X) but no paralysis of the tongue (XII). Breath-holds were from the end of a normal lung deflation and apparently from 100%O2. Fig. 2 shows that mean breath-hold duration was almost trebled. The absolute durations are also remarkable because breath-holds were only from end lung deflation (whereas those in Fig. 1 were from maximum lung inflation). It is also remarkable that breath-holding even remains possible without motor control of the glottis.
These experiments have been eclipsed because of the possibility that the prolongation was explained by blockade of carotid chemoreceptor afferents. But we now know from Fig. 1 that carotid nerve resection has no effect on breath-hold duration from maximal inflation with 100% O2. So the prolongation must be explained by something else. One possibility is that it was due to blockade of the pulmonary afferents in the vagus nerve that detect pulmonary inflation, deflation or irritation. But this cannot be the explanation either, because breath-hold duration is no different in patients with pulmonary vagus denervation (i.e. with lung transplantation) vs. either intact controls (Harty et al. 1996) or patients with heart transplantation (Flume et al. 1996). So apparently there remains another unidentified afferent in the vagus (or glossopharyngeal) nerves that makes an important contribution to the breakpoint. Of course Noble et al. (1970) only describe three subjects. No-one has ever confirmed their results (certainly with modern ethical concerns they never will!). Moreover, even this unidentified afferent does not provide a complete explanation, because breath-hold duration was only prolonged. Subjects still could not breath-hold to unconsciousness.
So how can it be that breath-hold duration is so affected by oxygen levels, yet the only chemoreceptors known to detect arterial hypoxia have little or no role in the breakpoint mechanism?
References
Cooper HE, Parkes MJ & Clutton-Brock TH (2003). CO2-dependent components of sinus arrhythmia from the start of breath-holding in Man. Am J Physiol 285, H841–H848.
Davidson JT, Whipp BJ, Wasserman K, Koyal SN & Lugliani R (1974). Role of carotid bodies in breath holding. N Engl J Med 290, 819–822.
Ferris EB, Engel GL, Stevens CD & Webb A (1946). Voluntary breath-holding III. J Clin Invest 25, 734–780.
Flume PA, Eldridge FL, Edwards LF & Mattison LE (1996). Relief of the ‘air hunger’ of breathholding. A role for pulmonary stretch receptors. Resp Physiol 103, 221–232.
Gross PM, Whipp BJ, Davidson JT, Koyal SN & Wasserman K (1976). Role of the carotid bodies in the heart rate response to breath holding in man. J Applied Physiol 41, 336–340.
Guz A (1966). Effects of blocking the vagus nerves in Man. In Breathlessness, eds. Howell JBM & Campbell EJM. pp. 65–71. Wiley-Blackwell, Oxford.
Guz A, Noble MIM, Widdicombe JG, Trenchard D & Mushin WW (1966a). Peripheral chemoreceptor block in man. Resp Physiol 1, 38–40.
Guz A, Noble MIM, Widdicombe JG, Trenchard D, Mushin WW & Makey AR (1966b). The role of vagal and glossopharyngeal afferent nerves in respiratory sensation, control of breathing and arterial pressure regulation in conscious man. Clin Sci 30, 161–170.
Harty HR, Mummery CJ Adams L, Banzett RB, Wright IG, Banner NR, Yacoub MH & Guz A (1996). Ventilatory relief of the sensation of the urge to breathe in humans: are pulmonary receptors important? J Physiol 490, 805–815.
Lin YC, Lally DA, Moore TA & Hong SK (1974). Physiological and conventional breath-hold break points. J Applied Physiol 37, 291–296.
Lugliani R, Whipp BJ, Seard C & Wasserman K (1971). Effect of bilateral carotid body resection on ventilatory control at rest and during exercise in Man. N Engl J Med 285, 1105–1112.
Noble MIM, Eisele JH, Trenchard D & Guz A (1970). Effect of selective peripheral nerve blocks on respiratory sensations. In Breathing: Hering-Breuer Centenary Symposium, ed. Porter R. pp. 233–247. J & A Churchill (Longmans Group), London.
Parkes MJ (2006). Breath-holding and its breakpoint. Exp Physiol 91, 1–15.
