Physiology News Magazine
From lamprey swimming to human stepping: evolutionary conservation of longitudinally-propagated spinal activity
Features
From lamprey swimming to human stepping: evolutionary conservation of longitudinally-propagated spinal activity
Features
Mélanie Falgairolle & Jean-René Cazalets
Universités Bordeaux 2 and 1, CNRS, Bordeaux, France
https://doi.org/10.36866/pn.69.15

A diverse array of biomechanical systems has evolved to satisfy locomotory requirements (flight, reptation, swimming, walking, etc.) within the animal kingdom’ s different environments. Effectively, the ability to move from one place to another requires reconciliation between conflicting behavioural objectives, in that the seemingly straightforward demand of an animal to actively move towards a target necessitates the disruption of the fragile postural equilibrium that has to be dynamically adjusted as body displacement proceeds. Thus the successful achievement of locomotor movement requires the integrated functioning of all body segments, including the fore- and hind-limbs, trunk, head, and the synergistic action of many other muscle groups to ensure the appropriate positioning of the different body regions.
From numerous studies on a variety of vertebrate organisms, it is now well established that the basic motor patterns underlying limb and trunk movements during locomotion are driven by central networks of neurons, so-called central pattern generators (CPGs), that for the foreand hind-limbs of quadruped animals are located within the lumbar and cervical spinal cord regions, respectively. In limbless anguilliform animals such as tadpoles, lamprey and snakes, body propulsion is driven by alternate leftright trunk muscle contractions that occur sequentially in an anterior-toposterior direction along the body length. The CPGs that control such rhythmic trunk bending have been shown to be segmentally-distributed along the whole spinal cord axis. In limbed vertebrates, however, very few studies have explored the role of trunk muscle contractions during locomotion, although a cyclic contraction of trunk muscles in time with rhythmic limb movements has been reported in various species (cat, rat and man).
We have recently begun to address a number of unresolved questions concerning the functional organization of spinal networks involved in trunk muscle activation during limb-based locomotion. What are the neural circuit substrates for coordinated hind-limb and trunk movements during actual locomotion in quadruped mammals, and to what extent have these neuronal networks been preserved during the course of evolution?
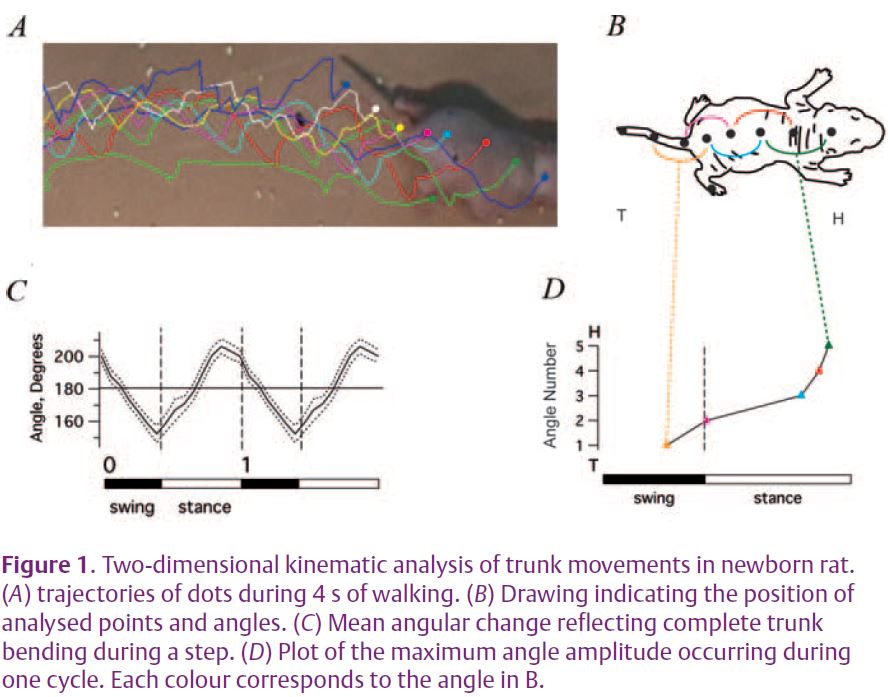
To explore the simultaneous functioning of the trunk and hindlimbs during actual locomotion, we have used both in vivo behavioural observations and in vitro electrophysiological approaches with the neonatal rat as our experimental model. In a first instance, to study coordinated back and tail movements during behaviour, rat pups were tagged with a series of ink dots placed along the dorsal axial line and were filmed during actual locomotion (Fig. 1A, 1B). By then determining the spatial coordinates of individual dots, regionally-specific body positions and angles could be computed. Since, the rat pup does not yet exhibit spontaneous locomotion at this age, forward progression was elicited by holding a tube containing nest material in front of the animal’s nose. As the animal moves towards the scent, the experimenter slowly withdraws the tube so that the animal follows it. Our 2D kinematic analysis showed that there is a rhythmic sequential change in trunk curvature during each step cycle of locomotor activity (Fig. 1C). Moreover, this rhythmic trunk bending is propagated from tail-to-head and the bending amplitude is larger in the lower back than in the upper thoracic body region.
We next sought to identify the origin and central distribution of spinal motoneurons supplying axial (trunk) musculature in the newborn rat. This was achieved by the intra-muscular injection of a retrograde marker (cholera toxin B subunit) which is taken up by motor axon terminals in the muscle and migrates into motoneuron cell bodies within the spinal cord over the ensuing 24 h. This revealed that motoneurons innervating the axial muscles are distributed along the entire spinal cord, from thoracic to coccygeal segments and including the lumbar segments where they co-localize with motoneurons that innervate the hind-limbs.
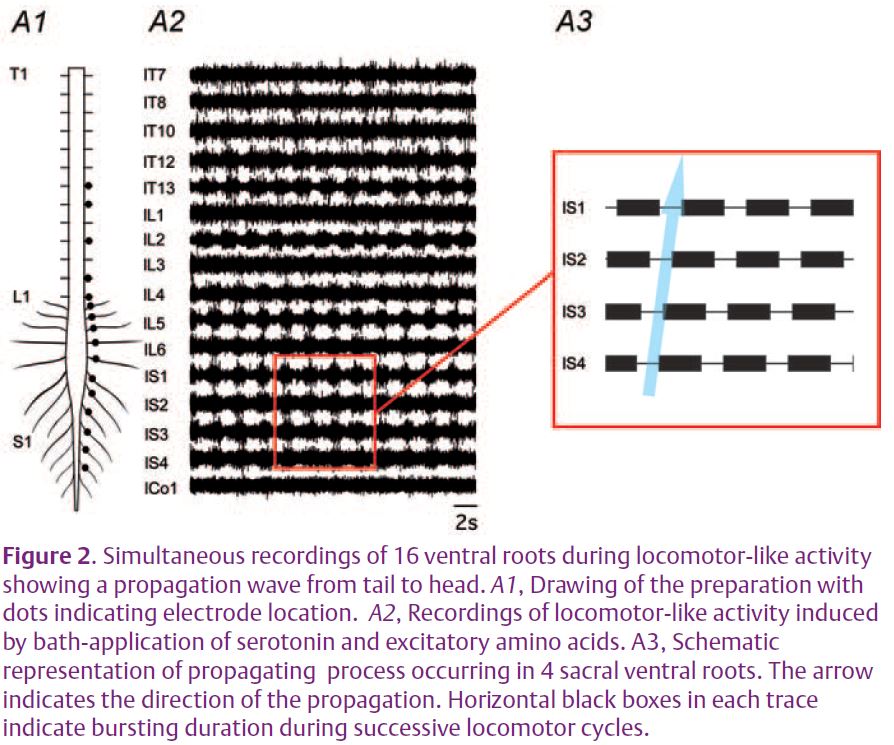
Finally, using isolated spinal cord preparations, we have analysed the functional interactions between the various cord regions during centrallygenerated motor activity in vitro. Sequences of ‘fictive’ locomotion, i.e. rhythmic locomotor-related activity produced in the absence of actual movement (since the spinal cord is physically isolated from muscles and sensory inputs), were elicited by the bath-application of a mixture of the monoamine, serotonin, and excitatory aminoacids (Cazalets et al. 1992). The core networks that generate hindlimb locomotor activity in rat were previously found to be located in lumbar segments L1 and L2 of the spinal cord (Cazalets et al. 1996). To assess the global functioning of spinal circuitry and to understand how the thoracic, lumbar and sacral segments interact, we recorded from up to 16 ventral motor roots simultaneously along the thoracolumbo-sacral spinal cord axis (Fig. 2A1, 2A2). A major finding from this approach is that waves of rhythmic activity are propagated sequentially to the various ventral roots along the cord from sacral (posterior) to upper (anterior) thoracic segments (Fig. 2A3). This intra-spinal discharge in which one cord region is activated after another, has been termed ‘metachronal’ motor activity. In vitro experiments where the isolated cord was sectioned at different levels have revealed three zones (thoracic, lumbar, sacral) in which the spinal motor networks possess the intrinsic capacity for generating rhythmic motor output. This approach also provided important insights into the underlying synaptic organization. First, rhythmic burst generation continued to occur in the longitudinally-sectioned hemi-spinal cord, indicating that the central circuitry does not require cross-cord connections to generate locomotorlike output. Second, based on the relative timing of motor bursts, it appears that the travelling wave can not be explained solely on the basis of axonal conduction velocities and synaptic transmission delays. Rather, it is likely that both long multisegmental neuronal pathways as well as local circuit interactions between adjacent segmental oscillators are involved in the coupling.
The data reported in our paper has allowed a functional diagram of interacting spinal networks to be proposed. In this model, the bursting properties of each spinal segment facilitate the transmission of motor activity along the spinal cord, while local intersegmental synaptic interactions also contribute to activity propagation. Cross-cord connections ensure bilateral coupling along the cord length, and long propriospinal pathways are also involved in coordination. The rostral part of the lumbar cord enlargement provides rhythmic locomotor-related output to rhythmic network elements in neighbouring segments that in turn convey and modulate output to their corresponding segmental motoneurons. In the intact spinal cord, the lumbar cord region probably imposes its own timing on the thoracic spinal cord generators, as it also does for the more rostral sacral segmental generators.
The first important conclusion of this study is that the rhythmic motor pattern that drives back muscle activation is centrally generated. Secondly, the metachronal nature of the propagating motor pattern is also centrally determined and remains strictly coordinated with hindlimb motor activity. Moreover, our data suggest that the networks responsible for the metachronal propagation of motor patterns during mammalian locomotion may correspond to those observed in lower vertebrates and even invertebrates, and thus appear to have been highly conserved during evolution. Interestingly, metachronal propagation is not only restricted to undulating or quadrupedal organisms. By investigating back muscle activity in humans we have recently found a comparable sequential activation during various modes of forward bipedal locomotion (manuscript in preparation). Presumably, such a ubiquitous organization serves to assist in dynamic postural adjustments to ensure fluent body and limb movements during locomotion.
References
Cazalets JR, Borde M & Clarac F (1996). The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci 16, 298–306.
Cazalets JR, Sqalli-Houssaini Y & Clarac F (1992). Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455, 187–204.
Falgairolle M & Cazalets JR (2007). Metachronal coupling between spinal neuronal networks during locomotor activity in newborn rat. J Physiol 580, 87–102.