Physiology News Magazine
Brain rhythms, synaptic plasticity and sleep
Features
Brain rhythms, synaptic plasticity and sleep
Features
Daniel Ulrich
Department of Physiology, Institute of Neuroscience, Trinity College, Dublin 2, Ireland
https://doi.org/10.36866/pn.69.18

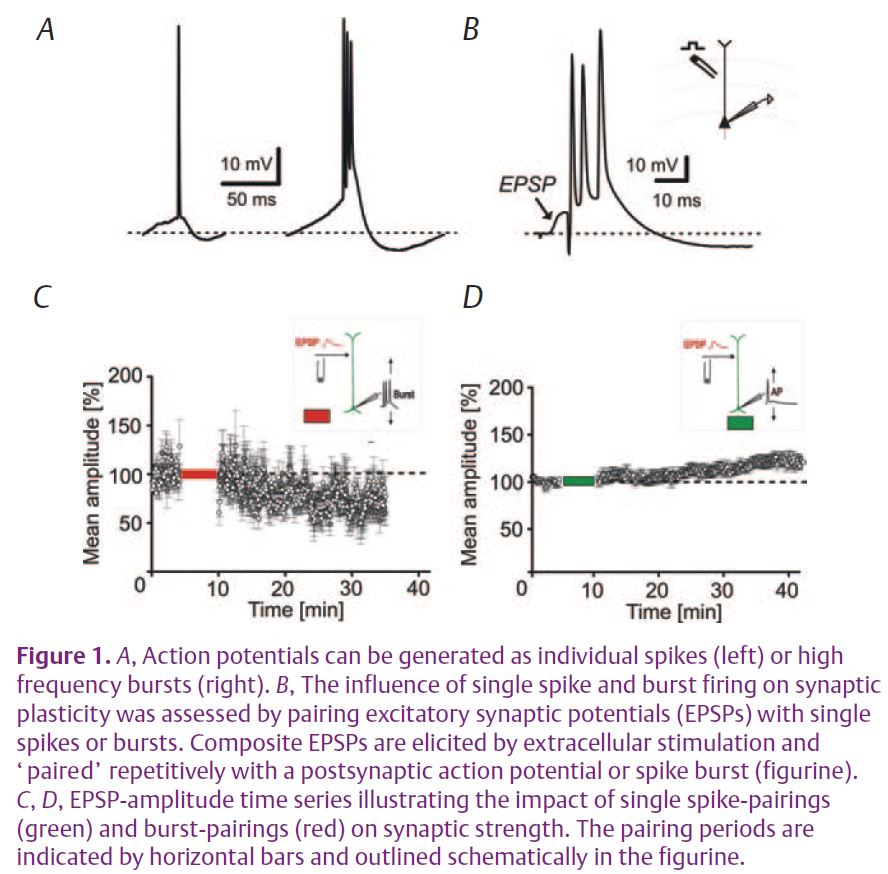
EEG recordings from the scalp disclose rhythmic electrical activity resulting from repetitive discharges of thousands of interconnected neurons in the forebrain. The frequency and synchrony of these rhythms change with the state of vigilance. During wakefulness and rapid-eye movement (REM) sleep fast oscillations (10–40 Hz) of low amplitude are prevalent, while during non-REM sleep slower rhythms (0.1–4 Hz) are highly synchronized over large areas of the forebrain. Associated with these state-dependent changes in global brain activity are character-istic alterations in the firing patterns of thalamic and cortical neurons (Steriade et al. 2001). During fast EEG rhythms, i.e. wakefulness and REM sleep, action potentials are generated as continuous trains of individual spikes (tonic mode), while during non-REM sleep they are emitted as high frequency bursts (burst mode) where action potentials are grouped together by a transient depolarizing potential (Fig. 1A).
While fast oscillations during wakefulness have been implicated in sensory processing and memory formation (Gray & Singer, 1989) the slow rhythms seem to play a role in reorganizing neural networks during sleep. There is accumulating evidence, mainly from behavioral studies, that both REM and non-REM sleep have an important influence on memory formation and learning (Walker & Stickgold, 2004). According to the ‘parallel ’ hypothesis, non-REM and REM sleep affect different types of memories, i.e. declarative and procedural, respectively. In the ‘sequential’ model all forms of memory are affected by both sleep episodes. The latter possibility would be in line with the alternating occurrence of non-REM and REM episodes during sleep and the fact that most experimental memory tasks inherently consist of a combination of different types.
It is widely accepted that changes in synaptic connectivity underlie memory formation and learning (Rioult-Pedotti et al. 2000). In particular, long-term potentiation (LTP) and depression (LTD) are longlasting increases or reductions in synaptic strength that are considered to be important cellular mechanisms underlying learning and memory formation. How, then, is synaptic strength influenced by the various discharge patterns observed during sleep?
Recent experiments in layer V pyramidal cells of rat somatosensory cortex in vitro have used a stimulation paradigm where excitatory synaptic potentials (EPSPs) were elicited in close temporal proximity with single action potentials or burst-discharges to mimic the near-synchronous activity of synaptically coupled neurons during wakefulness or sleep, respectively (Fig. 1B) (Birtoli & Ulrich, 2004). The results show that associations of EPSPs with single spikes predominately lead to LTP while associations of EPSPs with bursts induce an enduring reduction of the excitatory synaptic inputs (burst-LTD) (Fig. 1C, D). This suggests that firing patterns characteristic of wakefulness and sleep differentially affect synaptic plasticity. Pharmacological experiments revealed that while LTP was reliant on the NMDA (N-methylD-aspartate) subtype of glutamate receptors, burst-LTD depended on the concomitant activation of metabotropic group I glutamate receptors and low-threshold activated Ca2+ channels (Czarnecki et al. 2007) (Fig. 2A). The concurrent activation of both signalling pathways leads to a release of Ca2+ from internal stores and activation of protein kinase C (PKC). This enzyme promotes the endocytosis of AMPA receptors, which is the ultimate cause of a long-lasting depression of excitatory synaptic inputs (Fig. 2A). The concomitant requirement for presynaptic (i.e., neurotransmitter release) and postsynaptic (action potential bursts) activity explains the associative nature of burst-LTD (Birtoli & Ulrich, 2004).
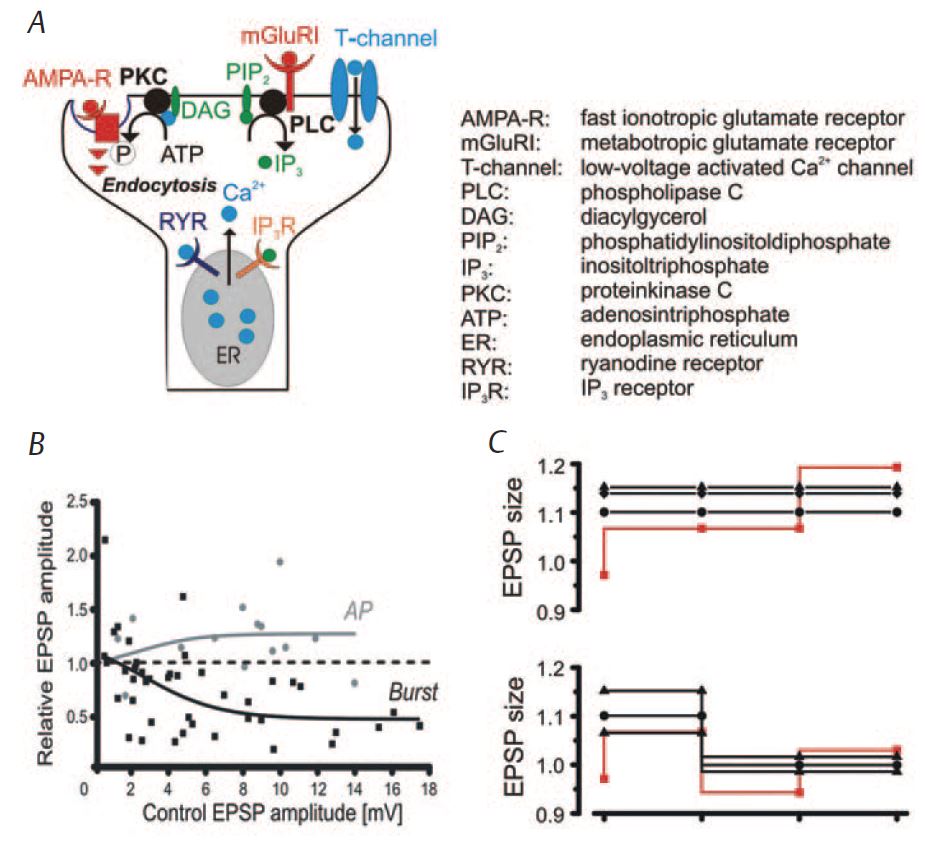
Further experiments revealed that the amount of LTP is proportional to the initial EPSP size, while burst-LTD was inversely related to the baseline amplitude of the EPSP (Fig. 2B). Downscaling of synaptic weights has been postulated as a major mechanism by which non-REM sleep contributes to memory consolidation (Tononi & Cirelli, 2003). Indeed, it was recently found that sleep-related improvement of a trained motor task is accompanied by an overall decrease of cortical spike activity (Fischer et al. 2005). This may indicate that memory improvement may be associated with a relative strengthening of particular connections by reducing the overall effectiveness of synaptic inputs. Indeed, preliminary data in vitro suggest that burst-LTD affects homoand hetero-synaptic inputs as expected for such a rescaling mechanism. Another potential important role of synaptic downscaling is to keep synaptic strengths within a physiological dynamic range. This would prevent synapses from being driven into saturation and thus allow their reuse over consecutive waking periods (Fig. 2C). It has been shown previously that LTP can be saturated and that this impedes further learning (Moser et al. 1998). Thus a synaptic rescaling process is likely to be of physiological relevance.
While we have shown that characteristic sleep/wake discharge patterns differentially influence synaptic strength, other factors like statedependent changes in neuromodulator concentrations are likely to affect synaptic plasticity as well.
Overall, there is growing evidence from different fields in neuroscience for a role of sleep in reorganizing neural circuits associated with memory formation. This would add another dimension to the function of sleep that is more traditionally believed to merely subserve energy replenishment.
References
Birtoli B & Ulrich D (2004). Firing modedependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci 24, 4935–4940.
Czarnecki A, Birtoli B, & Ulrich D (2007). Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol 578, 471–479.
Fischer S, Nitschke MF, Melchert UH, Erdmann C & Born J (2005). Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci 25, 11248–11255.
Gray CM & Singer W (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86, 1698–1702.
Moser EI, Krobert KA, Moser MB & Morris RG (1998). Impaired spatial learning after saturation of long-term potentiation. Science 281, 2038–2042.
Rioult-Pedotti MS, Friedman D & Donoghue JP (2000). Learning-induced LTP in neocortex. Science 290, 533–536.
Steriade M, Timofeev I & Grenier F (2001). Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85, 1969–1985.
Tononi G & Cirelli, C (2003). Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62, 143–150.
Walker MP & Stickgold, R (2004). Sleepdependent learning and memory consolidation. Neuron 44, 121–133.