Physiology News Magazine
The pattern of intracellular Ca2+ signal matters in epithelial secretion
Endocrine and exocrine cells respond to external cues by releasing different secretory molecules. Seung-Ryoung Jung and colleagues analyzed how ductal epithelia encode the external inputs into Ca2+ signals and decode them into specific types of secretion
Features
The pattern of intracellular Ca2+ signal matters in epithelial secretion
Endocrine and exocrine cells respond to external cues by releasing different secretory molecules. Seung-Ryoung Jung and colleagues analyzed how ductal epithelia encode the external inputs into Ca2+ signals and decode them into specific types of secretion
Features
Seung-Ryoung Jung (1), Toan D Nguyen (1,3), Bertil Hille (2), Duk-Su Koh (2,4)
Departments of 1: Medicine and:
2: Physiology and Biophysics, University of Washington, Seattle, WA, USA,
3: VA Puget Sound Health Care System, Seattle, USA
4: Department of Physics, POSTECH, Korea
https://doi.org/10.36866/pn.69.25

Calcium regulates many cellular processes including muscle contraction, fertilization, gene expression, membrane excitability, and exocytosis (Berridge et al. 2003). To modulate a variety of target molecules selectively in a cell, the Ca2+ signal itself has to carry discriminatory information. Sometimes selectivity is achieved by subcellular localization of the signal, and sometimes by the pattern of the signal. In electrically excitable cells, Ca2+ signals are typically determined by the activity of voltage-gated Ca2+ channels that mediate Ca2+ fluxes from the extracellular space into the cytoplasm. In non-excitable cells equipped with G-protein coupled receptor (GPCR)-linked IP3 generation, Ca2+ is often mobilized from internal stores by external agonists. The detailed time course of cytoplasmic free Ca2+ concentration ([Ca2+]i) is further modified by Ca2+ binding proteins and by transmembrane movements of Ca2+ ions between the cytoplasm, intracellular stores, other organelles, and the extracellular space. Ca2+ signals generated by GPCRs are often encoded as a graded increase of [Ca2+]i, with higher concentrations of agonists eliciting higher peak amplitudes of [Ca2+]i (‘amplitude’ modulation, AM). In addition, a signal can be conveyed by oscillations of [Ca2+]i, where agonist concentration determines the frequency of oscillations (‘frequency’ modulation, FM). The oscillation frequency seems to have a significant role in modulating gene expression (Dolmetsch et al. 1998) and certain enzymes, including Ca2+dependent intramitochondrial dehydrogenases and protein kinase C (Hajnóczky et al. 1995; Oancea & Meyer, 1998). In general, these Ca2+dependent enzymes are partially activated by each Ca2+ spike and then deactivated slowly as Ca2+ declines. If the next Ca2+ spike occurs before the enzyme is fully deactivated, there is a cumulative increase of enzyme activity.
Pancreatic ducts collect digestive enzymes released from acinar cells, delivering them to the duodenum. Pancreatic duct epithelial cells (PDEC) lining the ducts add an important component to the digestive fluid, HCO3 – (bicarbonate), which neutralizes acidic chyme from the stomach. Bicarbonate secretion from PDEC is mediated by ion channels and transporters (Nguyen et al. 1998). In addition, the cells secrete mucin, a major protein of the mucus layer, via exocytosis (Nguyen et al. 1998). Previous studies using PDEC monolayers demonstrated that [Ca2+]i elevations, induced by endogenous purinergic receptors (P2Y2 and P2Y11) promote both electrolyte (K+ and Cl–) and mucin secretion (Nguyen et al. 1998). However, the single-cell Ca2+ dynamics were not resolved in those studies which used populations of PDEC. Our recent single-cell measurements reveal a complex Ca2+ signaling system that depends on the agonist concentration: a low concentration of ATP or UTP (2 or 10 µM) evokes [Ca2+]i oscillations (Fig. 1B), whereas a high concentration of ATP or UTP (100 µM) evokes a sustained [Ca2+]i elevation (Jung et al. 2004, 2006). As measured with single-cell microamperometry, the sustained [Ca2+]i elevation stimulated exocytosis well, but the [Ca2+]i oscillations were much less efficacious (Fig. 1A), despite reaching similar peak [Ca2+]i levels (1-2 µM).
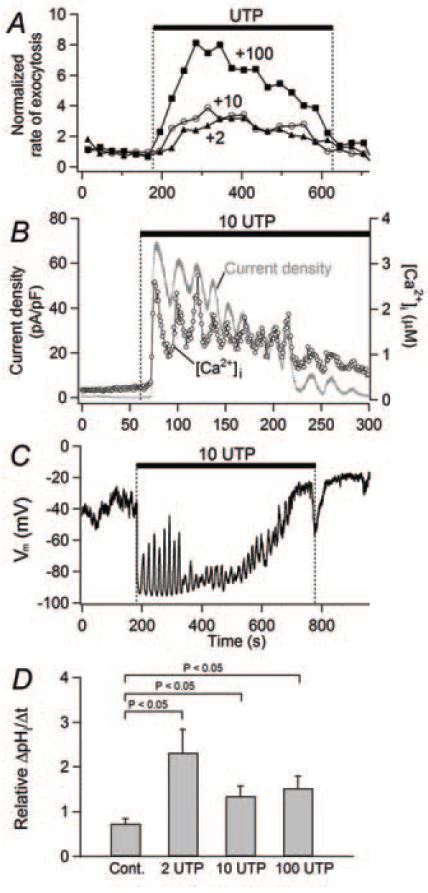
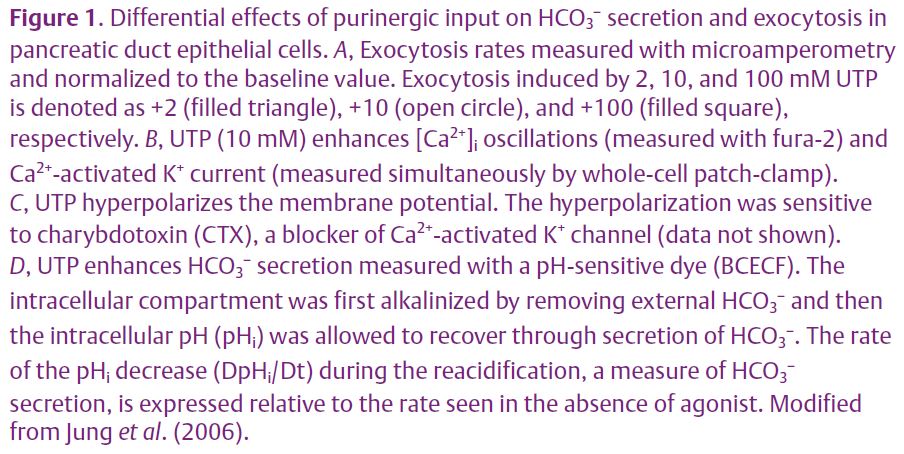
What is the role of oscillatory Ca2+ signals? We tested the hypothesis that Ca2+ oscillations open Ca2+activated K+ and Cl– channels expressed in PDEC. Both channels should be activated by micromolar levels of Ca2+. As anticipated, electrophysiological studies in the whole-cell patch configuration revealed a periodic activation of K+ channels synchronous with the [Ca2+]i oscillations (Fig. 1B) and periodic hyperpolarizations of the membrane (Fig. 1C). We identified the channel as a Ca2+-activated K+ channel of intermediate conductance (IK1/SK4 subtype) using pharmacology, single-channel recording, and immunohistochemistry. Finally, we reasoned that the hyperpolarization might accelerate secretion of HCO3 –. Bicarbonate secretion from single cells was assayed indirectly using a pH-sensitive dye (BCECF). A higher rate of intracellular pH decrease (∆pHi/∆t) should reflect increased HCO3– efflux from the cytoplasm to the extracellular compartment. We found that low (2 and 10 µM) and high (100 µM) concentrations of UTP stimulated HCO3– secretion to a similar extent, showing the equivalence of oscillatory and sustained [Ca2+]i increases in modulating HCO3 – secretion (Fig. 1D). Further, the UTP effect on HCO3– secretion was fully blocked by an IK channel blocker, charybdotoxin, suggesting that the K+ channel and its hyperpolarizing effect are essential for HCO3– secretion. The underlying mechanism(s) for the electrogenic HCO3 – secretion is not yet clear. The hyperpolarization could favor a lower intracellular Cl– concentration that would accelerate the activity of Cl–/HCO3 – exchanger indirectly. Alternatively, the hyperpolarization could increase HCO3– efflux through HCO3–permeable anion channels directly. An argument in favor of the Cl–/HCO3– exchanger was that blocking it with DIDS or by removing external Cl– eliminated the effect of UTP on HCO3 – secretion.
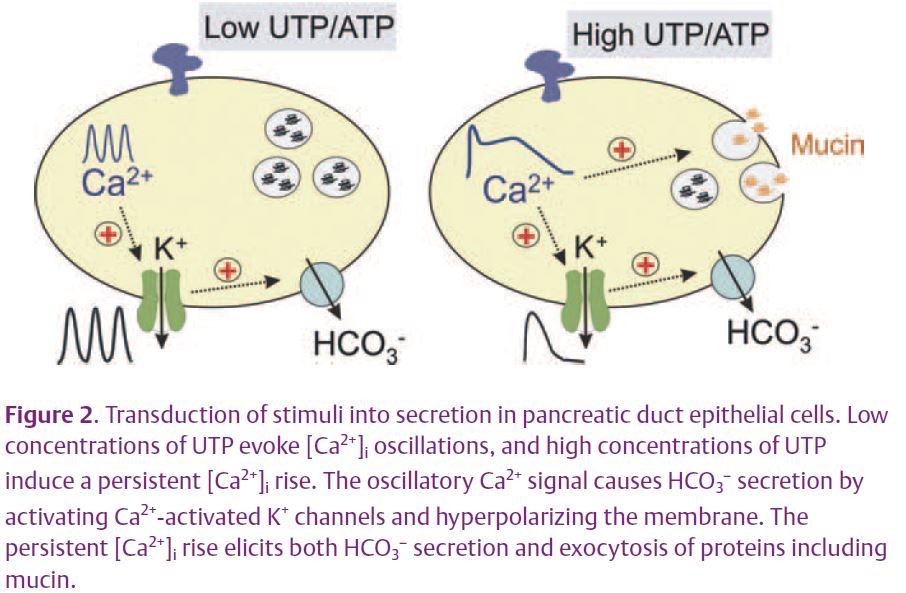
In summary, the intensity of external stimuli can be encoded into different patterns of [Ca2+]i increases, to be decoded later into different biological outputs (Fig. 2). Low concentrations of purinergic agonists evoke mainly HCO3– secretion, whereas high concentrations evoke mucin secretion as well. These differential responses would be particularly relevant to the emerging autocrine and paracrine function of ATP, the major physiological activator of these receptors. The extracellular concentration of ATP depends on ATP release, diffusion, dilution, and local metabolism. Further investigation should show whether other receptors coupled to Gq and PLC (e.g. histamine H1, protease activated (PAR-2), muscarinic, or cholecystokinin receptors) alone or in combination elicit similar responses.
Physiological significance of the ductal input-output relations
Along with neutralization of acidic chyme, the ductal HCO3 – probably also alters the rheologic properties of mucin secreted from the duct cells. The viscosity of mucin tends to increase at acidic pH. If secretion of HCO3 – is impaired, as in cystic fibrosis, the luminal pH would fall and mucin released from PDEC might become more viscous and not be cleared from the epithelial surface. Formation of a mucin gel in the pancreatic ductal tree could lead to the blockage of the small ducts and eventual destruction of the gland. We therefore suggest that HCO3 – and mucin secretion need to be well balanced. As UTP analogues have been advocated in the clinical treatment of cystic fibrosis, our findings suggest that low concentrations may be preferable to high concentration of these agents as they may increase HCO3 – secretion through activation of IK channels while stimulating less mucin production.
References
Berridge MJ, Bootman MD & Roderick HL (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4, 517–529.
Dolmetsch RE, Xu K & Lewis RS (1998). Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936.
Jung SR, Kim MH, Hille B, Nguyen TD & Koh DS (2004). Regulation of exocytosis by purinergic receptors in pancreatic duct epithelial cells. Am J Physiol 286, C573–579.
Jung SR, Kim KJ, Hille B, Nguyen TD & Koh DS (2006). Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. J Physiol 576, 163–178.
Nguyen TD, Moody MW, Savard CE & Lee SP (1998). Secretory effects of ATP on nontransformed dog pancreatic duct epithelial cells. Am J Physiol 275, G104–113.
Hajnóczky G, Robb-Gaspers LD, Seitz MB & Thomas AP (1995). Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424.
Oancea E & Meyer T (1998). Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 95 307-318.