Physiology News Magazine
Colonic elongation activates an intrinsic reflex that underlies slow transit and accommodation
It is generally believed that after-hyperpolarizing neurons are the only intrinsic sensory neurons in the gut wall. Recently, Smith and colleagues have demonstrated that some synaptic interneurons in the large bowel are activated by circumferential stretch and generate an ongoing motor activity even when AH neurons are silent. Further studies suggest that some descending interneurons are also mechanosensory since they are activated by longitudinal stretch rather than circumferential stretch. In particular, these mechanosensory neurons, which are NOS positive, respond to colonic elongation by releasing nitric oxide to reduce activity in intrinsic peristaltic circuits. This later reflex promotes storage by reducing the transit rate of fecal pellets down the large bowel
Features
Colonic elongation activates an intrinsic reflex that underlies slow transit and accommodation
It is generally believed that after-hyperpolarizing neurons are the only intrinsic sensory neurons in the gut wall. Recently, Smith and colleagues have demonstrated that some synaptic interneurons in the large bowel are activated by circumferential stretch and generate an ongoing motor activity even when AH neurons are silent. Further studies suggest that some descending interneurons are also mechanosensory since they are activated by longitudinal stretch rather than circumferential stretch. In particular, these mechanosensory neurons, which are NOS positive, respond to colonic elongation by releasing nitric oxide to reduce activity in intrinsic peristaltic circuits. This later reflex promotes storage by reducing the transit rate of fecal pellets down the large bowel
Features
Terence K Smith, Eamonn J Dickson, Grant W Hennig, Peter O Bayguinov, Nick J Spencer
Department of Physiology & Cell Biology, University of Nevada School of Medicine, Reno, Nevada, USA
https://doi.org/10.36866/pn.69.33
Transit of intraluminal contents through the human large bowel is extremely slow ( ≥ 30 hrs) compared to transit through other regions of the gastrointestinal tract. As intraluminal contents move down the large bowel, water and electrolytes are absorbed causing the contents to become more viscous leading to stool formation. This is dramatically illustrated in the guinea-pig large bowel, where the proximal colon contains viscous fluid chyme that is compacted into soft fecal pellets at the flexure between the proximal and distal colon (D’Antona et al. 2001).
A common view at the turn of the century was that an elongated colon (called dolichocolon) was a common cause of constipation. Although, this concept has been recently challenged (Muller-Lissner et al. 2005) it still persists in the literature: ‘… the cause of constipation was colonic slow-transit … which was always associated with dolichocolon’ (Ripetti et al. 2006). However, an excess production of nitric oxide (NO) within myenteric neurons has been proposed from immunohistochemical studies to have a role in the pathology underlying slow transit constipation in the human large bowel (Cortesini et al. 1995). Our laboratory has recently discovered a powerful intrinsic neural reflex that likely underlies colonic storage since it is activated by colonic elongation that triggers myenteric interneurons that release NO to depress motility (Dickson et al. 2007a, b).
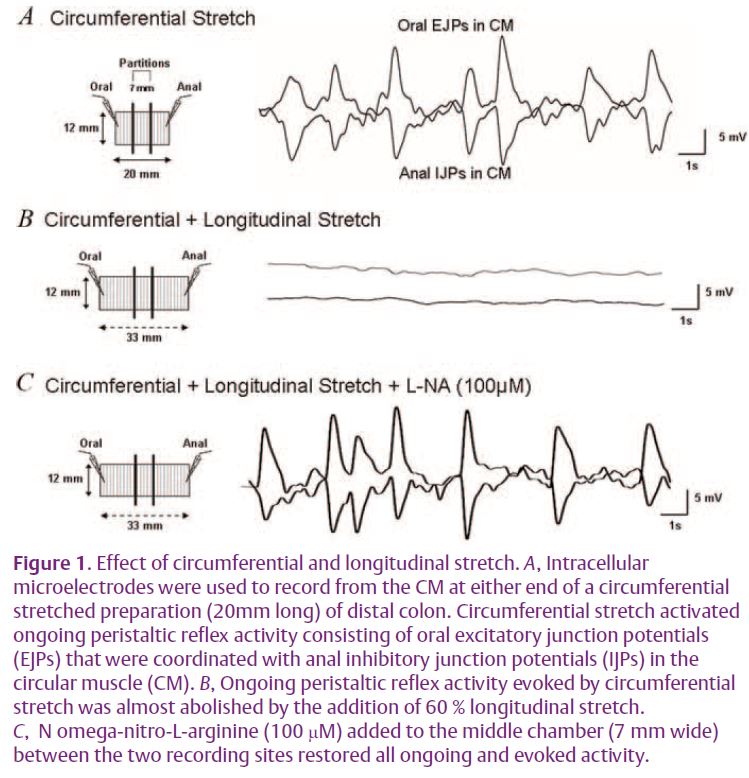
It is well known that there are two electrophysiological classes of myenteric neuron in the enteric nervous system of the large intestine, called originally AH and S type neurons by Hirst et al. (1974). After-hyperpolarizing (AH) neurons are rapidly adapting, multipolar neurons with one or two processes projecting down to the mucosa. They respond to chemical stimulation of the mucosa and smooth muscle tension (Furness et al. 1998; Smith, 1996; Spencer & Smith, 2004; Smith et al. 2005). In contrast, S (for synaptic) neurons are slowly adapting and receive fast excitatory postsynaptic potentials. They are uniaxonal excitatory and inhibitory motor neurons and ascending and descending interneurons (Lomax & Furness, 2000; Smith, 1996; Smith et al. 1992; Spencer & Smith, 2005). Some ascending and descending interneurons in the distal colon are stretch sensitive in that they detect circular muscle (CM) length rather than muscle tension (Smith et al. 2005; Spencer & Smith, 2004). In circumferentially stretched preparations of distal colon (~20mm long) these interneurons can generate ongoing peristaltic reflex activity even when AH neurons are silent (Spencer & Smith, 2004). This neural activity consists of excitatory junction potentials (EJPs) in the CM at the oral end that are phase locked in both time and amplitude with inhibitory junction potentials (IJPs) at the anal end of the preparation (Fig. 1A; Spencer & Smith, 2004). The only way this can occur is if the ascending and descending mechanosensory interneurons mediating this activity are synapsing with one another to form a reverberating circuit (Fig. 2; Smith et al. 2005; Spencer et al. 2006).
Immunohistochemical coding studies of myenteric neurons in the guinea-pig distal colon have revealed 3 chemically distinct classes of ascending interneurons and 4 classes of descending interneurons (Lomax & Furness, 2000). We were therefore curious as to whether more of these chemically distinct interneurons in the colon might be mechanosensory.
Over 40 years ago Hukuhara et al. (1960) described a reflex activated by longitudinal stretch. They found that stretching the canine small intestine in the longitudinal axis with a hemostat produced an active and transient relaxation of the CM that inhibited ongoing rhythmic contractions, which they referred to as the ‘muscular intrinsic inhibitory reflex’. They also found that removing the longitudinal muscle without apparently disrupting the myenteric plexus abolished this reflex, suggesting that the sensory elements mediating this reflex were in the longitudinal muscle (LM) layer.
Given this result, we were interested in determining the effects of longitudinal stretch on the ongoing peristaltic reflex activity generated by radial stretch of the guinea-pig distal colon (Spencer et al. 2002, 2006). Much to our surprise we found that the addition of longitudinal stretch did not produce a relaxation or an inhibitory junction potential in the muscle, but instead suppressed the ongoing oral EJPs and anal IJPs in the CM in a graded manner (Fig.1B; Dickson et al. 2007a, b). We referred to this reflex, which was quite different from that described by Hukuhara et al. (1960), as an intrinsic occult (hidden or obscure) reflex since it suppresses, rather than evokes, activity in the muscle. Furthermore, this suppression of activity by longitudinal stretch occurred even when the longitudinal muscle had been removed suggesting that the sensory processes of the neurons mediating this reflex were in the myenteric plexus or CM (Dickson et al. 2007a; Spencer et al. 2006). We were surprised to find that blocking NO synthesis between the oral and anal recording sites completely restored the amplitude and coordination of oral EJPs and anal IJPs in the CM, despite the maintained longitudinal stretch (Fig. 1C). This suggested that there were specific mechanosensory neurons that responded to longitudinal stretch by releasing NO to inhibit myenteric neurons in peristaltic reflex pathways (Fig. 2). From the chemical coding studies described above (Lomax & Furness, 2000), we reasoned that the neurons responding to longitudinal stretch were most likely descending NOS +ve interneurons, since the other interneurons contained mainly ACh and potential peptidergic neurotransmitters that are mainly excitatory to other myenteric neurons.
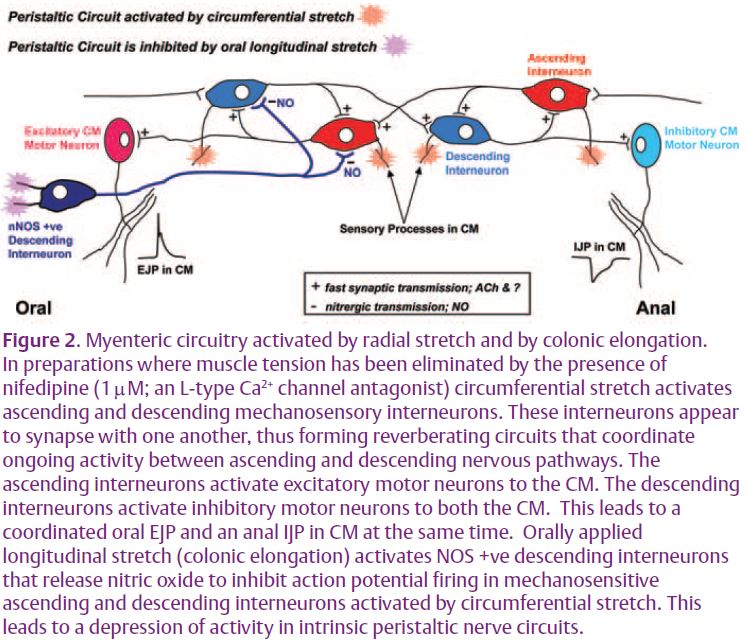
Calcium imaging followed by immunohistochemistry of myenteric neurons in preparations that had been stretched in both axes to inhibit neurons in peristaltic nerve pathways revealed ongoing activity in a class of neurons that were anally projecting NOS +ve neurons (Dickson et al. 2007a). Since we had also blocked synaptic transmission in these preparations with a variety of drugs we reasoned that these must be mechanosensitive interneurons responding to colonic elongation, rather than inhibitory motor neurons. Intracellular microelectrode studies revealed that NO mediated inhibitory postsynaptic potentials (IPSPs) could be evoked in mechanosensory interneurons. The IPSPs decreased action potential firing in these interneurons and thus prevented them from activating excitatory and inhibitory motor neurons to the muscle. Later studies confirmed that this occult reflex was polarized since oral, but not anal, longitudinal stretch inhibited junction potential activity and pellet propulsion (Dickson et al. 2007a, b).
When the guinea-pig distal colon is removed from the animal it is normally full of fecal pellets. Under these circumstances fecal pellet evacuation is slow and sporadic taking ~30 mins to empty all pellets compared to approximately a minute for propulsion of a single pellet down an empty segment of distal colon (D’Antona et al. 2001). Interestingly, we found that when the isolated colon is full of pellets it is elongated by about 40% compared to its length following the expulsion of pellets (Smith et al. our unpublished observations). Therefore, it would seem highly plausible to assume that the elongation of the colon produced by the pellets activates the occult reflex that slows pellet propulsion and promotes storage.
Our studies have revealed that, in addition to AH sensory neurons, distinct interneurons in the large bowel are mechanosensory and respond to either circumferential stretch or longitudinal stretch. We have described an intrinsic occult reflex that is activated by colonic elongation. Colonic elongation appears to inhibit colonic motility by activating NOS +ve descending interneurons that release NO to suppress activity in mechanosensitive interneurons involved in peristalsis. Clinically, the intrinsic occult reflex provides a negative feedback system that would be expected to exacerbate the inhibition of motility associated with constipation in an impacted colon. It remains to be seen whether any more myenteric interneurons in the colon are also mechanosensory.
Acknowledgements
This study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases: RO1 DK45713 (TKS).
References
Cortesini C, Cianchi F, Infantino A & Lise M (1995). Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci 40, 2450-2455.
D ‘Antona G, Hennig GW, Costa M, Humphreys CM & Brookes SJ (2001). Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastro Motil 13, 483-492.
Dickson E, Spencer NJ, Hennig GW, Bayguinov O, Ren J, Heredia D & Smith TK (2007a). An intrinsic occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132, 1912-24.
Dickson E, Ren, Hennig GW, Spencer NJ & Smith TK (2007b). A polarized intrinsic occult reflex underlies accommodation and slow transit in the large bowel. Gastroenterology A247, S1688.
Furness JB, Kunze WA, Bertrand PP, Clerc N & Bornstein JC (1998). Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54, 1-18.
Hirst GDS, Holman ME & Spence I (1974). Two types of neurons in the myenteric plexus of duodenum in the guinea-pig. J Physiol 236, 303–326.
Hukuhara T, Nakayama S & Nanba R (1960). Locality of receptors concerned with the intestino-intestinal muscular intrinsic reflexes Jap J Physiol 10, 414-418.
Lomax AE & Furness JB (2000). Neurochemical classification of enteric neurons in the guineapig distal colon. Cell Tissue Res 302, 59-72.
Muller-Lissner SA, Kamm MA, Scarpignato C & Wald A (2005). Myths and misconceptions about chronic constipation. Am J Gastroenterol. 100, 232-242.
Ripetti V, Caputo D, Greco S, Alloni R & Coppola R (2006). Is total colectomy the right choice in intractable slow-transit constipation? Surgery 140, 435-440.
Smith TK (1996). An electrophysiological identification of intrinsic sensory neurons that are excited by 5-HT applied to the mucosa in the guinea-pig proximal colon. J Physiol 495, 102P.
Smith TK, Bornstein JC & Furness JB (1992). Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. J Neurosci 12, 1502–1510.
Smith TK, Hennig GW & Spencer NJ (2005). Mechanosensory transduction in the enteric nervous system. Physiology News 58, 23–25.
Spencer NJ, Hennig GW & Smith TK (2002). A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545, 629–648.
Spencer NJ & Smith TK (2004). Mechanosensory S-neurons rather than AH neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol 558, 577–596.
Spencer NJ, Dickson EJ, Hennig GW & Smith TK (2006) Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guineapig distal colon. J Physiol 576, 519-531.