
Physiology News Magazine
Limitation to exercise performance at altitude – where is peripheral muscle fatigue important?
You might think the answer is ‘where it hurts’, but there is much more to it. Markus Amann explains
Features
Limitation to exercise performance at altitude – where is peripheral muscle fatigue important?
You might think the answer is ‘where it hurts’, but there is much more to it. Markus Amann explains
Features
Markus Amann
The John Rankin Laboratory of Pulmonary Medicine, University of Wisconsin-Madison Medical School, USA
https://doi.org/10.36866/pn.68.28

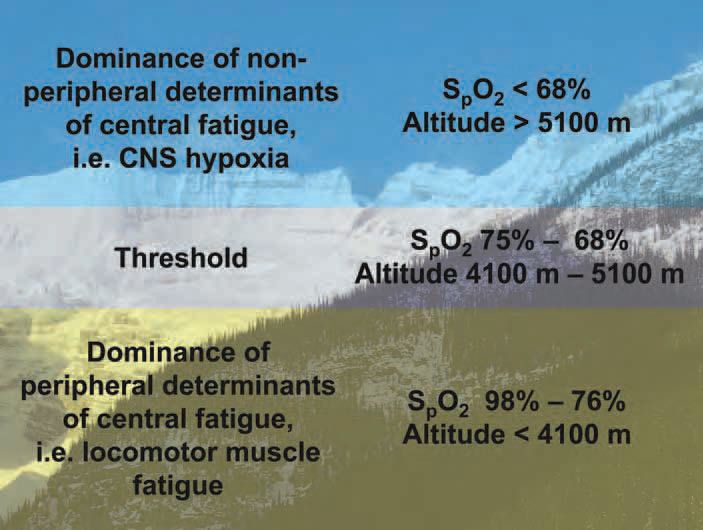
We have previously proposed that neural afferent feedback associated with peripheral locomotor muscle fatigue exerts an inhibitory influence on the central motor drive resulting in a centrally mediated limitation of exercise. This regulatory mechanism appears to be valid from sea level up to moderate altitudes (Amann et al. 2006a; Romer et al. 2007). More recently we have shown that the magnitude of this inhibitory peripheral feedback limiting central motor drive is significantly less at exhaustion in severe hypoxia, although exercise performance is limited even more (Amann et al. 2007). These observations suggest that, beyond moderate altitudes, other sources of inhibition of central motor drive start to outweigh the limiting effects of peripheral muscle fatigue and its associated inhibitory feedback (Fig. 1).
However, before going into further details of our newly proposed fatigue theorem, we will take a few steps back to recapitulate and reflect on some of our earlier findings. Based on strong correlative data, we think that sensory afferent feedback, originating in the fatiguing locomotor muscle, back to the CNS is a key determinant of the conscious (and/or subconscious) regulation of central motor drive (i.e. exercise performance). We believe in a strong link between ‘peripheral’ (i.e. biochemical changes within the muscle) and ‘central’ fatigue (reductions in CNS motor drive to the working muscle) (Amann et al. 2006a). Furthermore, we think that the magnitude of this inhibitory neural feedback is proportional to the magnitude of peripheral locomotor muscle fatigue, which in turn is highly sensitive to arterial O2 content (CaO2) (Amann et al. 2006b), and consequently acts as a dose-dependent trigger of central fatigue. In lay terms: the lower the O2 transport to the working legs during exercise the faster your locomotor muscle fatigue, the greater the inhibitory feedback to the CNS, the less the magnitude of central motor command and the greater the limitation to exercise performance.
The purpose of such a regulatory feedback mechanism is presumably to protect the muscle from an ‘excessive’ development of peripheral fatigue beyond a critical threshold or ‘sensory tolerance limit’ (Gandevia, 2001). Based on a series of experiments during which we altered the inspiratory O2 fraction (FIO2) to simulate various altitudes, we demonstrated that this regulatory mechanism is crucial in hyperoxia and up to a level of acute hypoxia equivalent to an altitude of approximately 4,000 m (Amann et al 2006a; Romer et al. 2007). After these initial studies, the following questions arose naturally: what happens if the mountaineer continues to climb beyond this altitude? Is the exercise-induced magnitude of peripheral locomotor muscle fatigue and/or the rate of development of fatigue of these muscles still as important a regulated variable at extreme altitudes? Or does the priority change and other organ systems, like the brain, take over the hierarchy of regulated variables due to the increased threat associated with severe systemic hypoxemia induced by exercise beyond moderate altitudes?
Previous reports in the literature have already implied that severe CNS hypoxia may result in inhibitory effects on central motor drive (Kayser et al. 1994; Calbet et al. 2003). These conclusions were drawn from the observation that after cycling to exhaustion at great altitudes (> 5,000 m) the administration of 100% O2 – thereby reversing the arterial desaturation –enabled the subjects to continue to exercise. Ultimately, these findings argue against the validity of our proposed regulatory mechanism (Amann et al. 2006a) (the development of central fatigue based on peripheral feedback) in extreme altitudes and indicate the existence of fast responding hypoxia-sensitive sources of inhibition of central motor drive. Now, how can our recently proposed regulatory mechanism and the indications of those ‘re-oxygenation’ experiments be tied together – is there a link? Is there a fluctuating impact on the development of central fatigue? How is the magnitude of the impact of each of these determinants of central fatigue regulated?
We have suggested that the relative effects of centrally versus peripherally originating impairments of central motor drive (i.e. limitations in exercise performance) change with the level of convective O2 transport as affected by acute hypoxia (Amann et al. 2007). Subjects were instructed to pedal against a heavy intensity fixed workload to task failure in normoxia, moderate and severe hypoxia (FIO2 0.21, 0.15, and 0.10, respectively). Clear criteria for task failure (drop in pedal cadence below 70% of self-selected target cadence for ≥5 s) and exhaustion (drop in pedal cadence below 60% of self-selected target cadence for ≥5 s) were established prior to the study. When the subjects, unaware of the procedure, reached task failure in each condition, arterial hypoxemia was rapidly removed by surreptitiously switching to an FIO2 of 0.3 (re-oxygenation). A significant prolongation of exercise time to exhaustion was not achieved following re-oxygenation at task failure in normoxia [arterial hemoglobin saturation (SpO2) of ~94%] and moderate hypoxia (SpO2 ~82%). However, in severe hypoxia (SpO2 ~67%), re-oxygenation at task failure elicited a significant prolongation (+170 %!) of time to exhaustion.
Why this difference with severe hypoxia? At task failure in normoxia and moderate hypoxia peripheral locomotor muscle fatigue – assessed via changes in quadriceps twitch force (∆Qtw) as measured pre- versus post-exercise in response to supramaximal femoral nerve stimulation – has reached the individual critical threshold (∆Qtw from pre- to post-exercise of about –36%). As expected, this magnitude of peripheral fatigue did not change further within the additional few seconds of exercise to exhaustion after re-oxygenation following either normoxia or moderate hypoxia. This is consistent with the literature indicating that re-oxygenation has no instant alleviating effect on the already induced magnitude of peripheral muscle fatigue. Interestingly, however, at task failure in severe hypoxia peripheral muscle fatigue was significant but only about two-thirds of the level of fatigue measured at task failure in normoxia and moderate hypoxia and therefore far below the individual threshold or sensory tolerance limit. Following re-oxygenation in severe hypoxia, subjects continued to exercise and peripheral fatigue continued to develop to the same level (critical threshold) as observed at exhaustion in normoxia and moderate hypoxia (∆Qtw about –36%).
So, what limits endurance exercise in normoxia and moderate hypoxia vs. severe hypoxia and why was there significantly less locomotor muscle fatigue at task failure in severe hypoxia? The data indicate that exercise- and altitude-induced arterial hypoxemia as experienced at sea level and up to moderate altitudes (~ 4100 m), per se, is not severe enough – by itself – to impose an inhibitory influence on central motor drive in healthy humans. The CNS at these altitudes ‘allows’ the development of peripheral locomotor muscle fatigue until the individual critical threshold – or sensory tolerance limit – is achieved. This then in turn curtails central motor output, presumably via strong inhibitory neural feedback to the CNS.
At severe altitudes (> 5100 m) the roles seem to be reversed. The level of arterial hypoxemia during exercise at these extreme altitudes imposes a severe threat to brain function itself, possibly operating via interference with cerebral neurotransmitter turnover. Accordingly, central motor output is constrained largely independent from any inhibitory afferent feedback originating in the periphery. This central inhibitory effect of severe hypoxia probably serves to avoid severe cerebral dysfunction far in advance of reaching the individual critical threshold of peripheral muscle fatigue.
Why is end-exercise peripheral muscle fatigue at exhaustion (following re-oxygenation at task failure) identical in all three conditions? At sea level and at moderate altitude, peripheral locomotor muscle fatigue rises all the way to the individual critical threshold. Exercise is thus, despite re-oxygenation at task failure, terminated via a reduction in central motor output to prevent further development of peripheral fatigue beyond the individual critical threshold. By reversing the arterial hypoxemia and CNS hypoxia at task failure in severe hypoxia, the constraint to exercise quickly vanishes, exercise can be continued and the locomotor muscles continue to accumulate fatigue until the individual critical threshold is reached.
To date we only have correlative data to support the role of peripheral muscle fatigue and associated inhibitory feedback on central motor drive. We are currently investigating the effects of a direct blockade of neural feedback originating in the exercising and fatiguing locomotor muscles on central motor drive and the development of peripheral muscle fatigue.
In summary, we believe the current data support the concept that the dominance of CNS hypoxia over peripheral muscle fatigue in influencing central motor output, and therefore exercise performance, occurs below a threshold level of acutely compromised O2 transport. This level is represented by a range of 75–68% SaO2, or the corresponding acute exposure to altitudes of about 4100–5100 m.
References
Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF & Dempsey JA (2006a). Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue. J Physiol 575, 937–952.
Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ & Dempsey JA (2006b). Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol 101, 119–127.
Amann M, Romer LM, Subudhi AW, Pegelow DF & Dempsey JA (2007). Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581, 389–403.
Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD & Saltin B (2003). Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284, R291–303.
Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725–1789.
Kayser B, Narici M, Binzoni T, Grassi B & Cerretelli P (1994). Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J Appl Physiol 76, 634–640.
Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF & Dempsey JA (2007). Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol 292, R598–606.
