
Physiology News Magazine
Neuromechanical matching of central respiratory drive: a new principle of motor unit recruitment?
Features
Neuromechanical matching of central respiratory drive: a new principle of motor unit recruitment?
Features
Jane E Butler (1), André De Troyer (2), Simon C Gandevia (1), Robert B Gorman (1), Anna L Hudson (1)
1: Prince of Wales Medical Research Institute and the University of New South Wales, Sydney, Australia
2: Laboratory of Cardiorespiratory Physiology, Brussels School of Medicine Unit and Chest Service, Erasme University Hospital, Brussels, Belgium
https://doi.org/10.36866/pn.67.22

Compared with the muscles involved in human dexterity or those involved in locomotion, the muscles responsible for ventilation receive little attention. Inspiration is achieved largely by contraction and shortening of the diaphragm and expiration is largely passive during ‘quiet’ breathing. The muscles lying between the ribs are easily ignored: they come in layers and the angulation of their muscle fascicles varies topographically along an intercostal space. Apart from the diaphragm, the function of the respiratory muscles was a subject of debate for many years (Campbell et al. 1970). Yet, it is now clear from careful electromyographic studies, that several other muscles acting on the chest wall contract rhythmically during quiet breathing – these include the scalene muscles, the parasternal intercostal muscles and the external intercostal muscles. Hence, there is a group of ‘obligate’ inspiratory muscles in quiet breathing. At the other extreme, if respiratory drive becomes sufficiently high, for example with a high concentration of inspired carbon dioxide, central respiratory drive potentials are observed in hindlimb motoneurones (Kirkwood et al. 2005).
Based on the Maxwell reciprocity theorem, Wilson & De Troyer (1992) showed that the mechanical advantage of a particular muscle acting on the chest wall (i.e. the change in pleural pressure produced by the muscle per unit muscle mass) could be assessed by measuring the length change of the muscle during inflation of the respiratory system. Thus, a muscle with a high mechanical advantage shortens more than a muscle with a low mechanical advantage. Using this method, these investigators then measured the distribution of mechanical advantage among the intercostal muscles in the different interspaces (De Troyer et al. 2005).
They subsequently evaluated the distribution of neural drive among these muscles during spontaneous breathing in anesthetized dogs, and these studies showed that muscles with a low mechanical advantage for inspiration become active relatively late in inspiration and contract weakly, whereas those with a high mechanical advantage become active early in inspiration and contract strongly. This immediately raises two questions: how is such a scheme organized to match the topographic drive to respiratory effectiveness in different regions of muscle, and, does such a scheme operate in awake animals and in humans? In dogs, this matching mechanism contains a ‘hard-wired’ element because it persists after denervation of the diaphragm and the chest wall (De Troyer et al. 2005). Hence, while afferents from these respiratory structures can influence inspiration, their activity is not necessary to generate a neural output that contains some ‘neuromechanical matching’.
We applied methods of motor unit sampling that we had previously used to record from populations of motor units in healthy subjects and patients with respiratory disease (e.g. De Troyer et al. 1997), to determine how the central nervous system drove the intercostal muscles during quiet breathing in awake seated humans (De Troyer et al. 2003; Gandevia et al. 2006). With the assistance of ultrasound and auditory feedback to the experimenter, monopolar recording electrodes are manipulated within regions of the intercostal muscles to record the activity of many single units. The timing and rate of their discharge is calculated. There are major topographical differences in the activity of motor units in the external intercostal muscles in quiet breathing: motor unit activity occurs early in inspiration in the 3rd dorsal external intercostal (after an average of ∼10% of inspiratory time), but it occurs later in the 5th dorsal external intercostal (after about a third of inspiratory time) and is usually absent in the 7th dorsal external intercostal. The level of peak inspiratory motor unit discharge has a corresponding gradient: higher firing rates in the 3rd dorsal external intercostal compared with lower ones in the 5th dorsal external intercostal (11.9 ± 0.3 Hz versus 6.0 ± 0.5 Hz; mean ± SEM). Units discharging tonically, but still with an inspiratory modulation, are more frequent in the lower dorsal external intercostal spaces.
We then applied this approach to the full set of 5 parasternal intercostal muscles, with all spaces being studied in one session. Again, a marked topographical distribution of inspiratory activity occurs with a similar cranio-caudal gradient. Activity in the first three parasternal intercostals commences after ∼10% inspiratory time, with an average peak single motor unit discharge of 13.4 ± 1.0 Hz in the first space (Fig. 1). However, activity starts after about a third of the inspiratory time in the lower two spaces and single motor unit discharge reaches only 8.0 ± 1.0 Hz in the 5th parasternal intercostal. Again, tonic activity is prominent in the lower spaces where inspiratory mechanical advantage is lower (Fig. 1B).
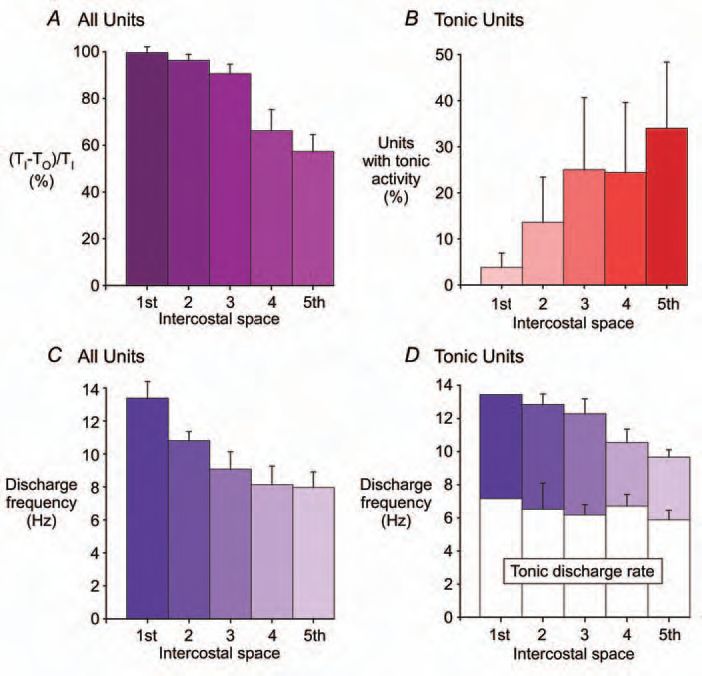
These results are intriguing for several reasons. First, within some spaces, such as the 3rd external intercostal space, there is also a diminution in the mechanical advantage along the intercostal space, from posterior to anterior, and this is paralleled by a reduction in neural drive (Fig. 2A). If, as has robustly been shown, there is an orderly recruitment of motoneurones according to Henneman’s size principle (Henneman, 1957), then another principle may be superimposed (Fig. 2B). This would be recruitment according to the mechanical advantage of the muscle fibres innervated by the motoneurone. How and why would such a matching strategy be laid down in development? Does this principle of neuromechanical matching occur for structures apart from those in the trunk where muscles have a large lateral dimension relative to the lengths of their muscle tendon units? Is it adaptable?
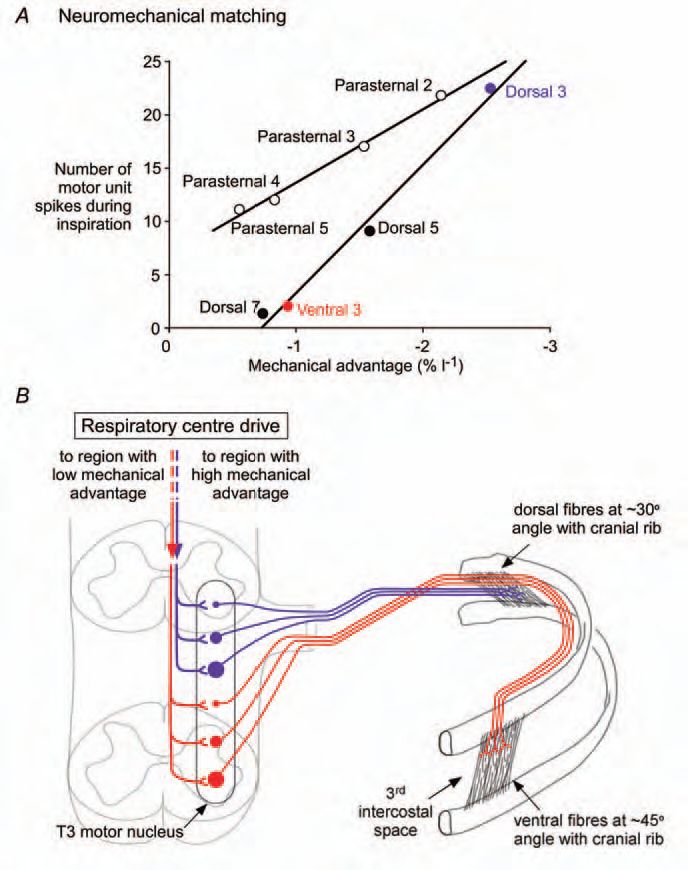
Second, how might the descending drives from the medulla achieve the neuromechanical matching? The topographic and temporal disparities among the intercostals are unlikely to be achieved by the motoneurones receiving a near-synchronous descending drive (via ‘central respiratory drive potentials’). Some motoneurones firing tonically do not increase their inspiratory discharge until after many of their neighbours have begun to discharge phasically. This implies that the temporal dispersion in the activation times within a subset of motoneurones innervating a particular region of an intercostal muscle may reflect dispersion in timing of the descending inspiratory drive or sculpting of descending drive at a spinal level. Further, we do not yet know if similar neuromechanical matching accompanies voluntary inspiratory efforts.
Finally, the results highlight how little we know about the total daily activity patterns of the intercostal muscles. Their function includes trunk movement as well as phasic and tonic stabilization of the spine associated with upper and lower limb movement. This may explain why the very limited data on fibre-type for these muscles does not seem to match their activity patterns during quiet breathing, and why their inspiratory activity may be governed by a unique strategy.
References
Campbell EJ, Agostoni E & Newsom Davis J (1970). The respiratory muscles: mechanics and neural control. Lloyd-Luke (Medical Books) Ltd, London.
De Troyer A, Gorman RB & Gandevia SC (2003). Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol 546, 943-954.
De Troyer A, Kirkwood PA & Wilson TA (2005). Respiratory action of the intercostal muscles. Physiol Rev 85, 717-756.
De Troyer A, Leeper JB, McKenzie DK & Gandevia SC (1997). Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med 155, 1335-1340.
Gandevia SC, Hudson AL, Gorman RB, Butler JE & De Troyer A (2006). Spatial distribution of inspiratory drive to the parasternal intercostal muscles in humans. J Physiol 73, 263-275.
Henneman E (1957). Relation between size of neurons and their susceptibility to discharge. Science 126, 1345-1347.
Kirkwood PA, Denton ME, Wienecke J, Nielsen JB & Hultborn H (2005). Physiological roles for persistent inward currents in motoneurones: insights from the central respiratory drive. Biocybernetics and Biomedical Engineering 25, 31-38.
Wilson TA & De Troyer A (1992). Effect of respiratory muscle tension on lung volume. J Appl Physiol 73, 2283-2288.
